FLT3-inhibitor therapy for prevention and treatment of relapse after allogeneic hematopoietic cell transplantation
- PMID: 35460465
- PMCID: PMC9392688
- DOI: 10.1007/s12185-022-03352-6
FLT3-inhibitor therapy for prevention and treatment of relapse after allogeneic hematopoietic cell transplantation
Abstract
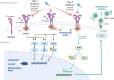
The curative potential of allogeneic hematopoietic cell transplantation (allo-HCT) for acute myeloid leukemia (AML) relies on the graft-versus-leukemia (GVL)-effect. Relapse after allo-HCT occurs in a considerable proportion of patients, and has a dismal prognosis with very limited curative potential, especially for patients with FLT-ITD-mutated AML. Since the first description of sorafenib for treatment of FLT3-ITD-mutated AML, several clinical trials have tried to determine the efficacy of FLT3 inhibitors for preventing and treating AML relapse after allo-HSCT, but many questions regarding differences among compounds and mechanisms of action remain unanswered. This review provides an overview on the established and evolving use of FLT3 inhibitors to prevent or treat relapse of AML in the context of allo-HCT, focusing on the recently discovered immunogenic potential of some FLT3 inhibitors and addressing the possible mechanisms of leukemia drug-escape.
Keywords: Acute myeloid leukemia; Allogeneic hematopoietic stem cell transplantation; FLT3-ITD; Gilteritinib; Midostaurin; Relapse; Sorafebin.
© 2022. The Author(s).
Conflict of interest statement
F.B. has no conflict of interest to declare. R.Z. received honoraria from Novartis, Sanofi and Mallinckrodt outside of the submitted work.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

