Impact of right ventricular pacing site on the subcutaneous ICD sensing-a step towards personalised device therapy?
- PMID: 35460502
- PMCID: PMC11832633
- DOI: 10.1007/s10840-022-01218-9
Impact of right ventricular pacing site on the subcutaneous ICD sensing-a step towards personalised device therapy?
Abstract
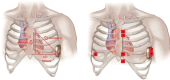
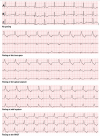
Background: Patients with an existing subcutaneous implantable cardiac defibrillator (S-ICD) may develop a pacing indication. When transvenous pacing is not feasible, combining an S-ICD and a leadless pacemaker (LP) can be a reasonable option. There are reports of concomitant use of both devices. However, the effect of pacing on the S-ICD sensing is not well studied. We hypothesise that pacing changes R and T-wave amplitudes, causing changes in R:T ratios as perceived by a S-ICD, increasing the risk for T wave oversensing (TWO) during paced rhythm with a subsequent risk of inappropriate shocks.
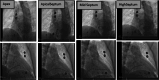
Methods: This is a prospective study in patients undergoing electrophysiological studies. Participants were fitted with a Holter®, and the leads were placed to correspond to the vectors of an S-ICD. The right ventricle was paced at four positions for 10 beats each at 8 mA/2 ms. The Holter® traces were analysed, using two-way analysis of variance (ANOVA) to assess the effect of pacing on the R:T ratio.
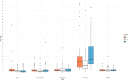
Results: Forty-seven patients (age 56.02 ± 16.02, 72% male) were enrolled (81% structurally normal heart, 15% dilated cardiomyopathy, 2% ischaemic cardiomyopathy, and 2% adult congenital heart disease). Age, sex, and aetiology had no effect on the R:T ratio. Pacing caused significant changes in the R:T ratio. There was no significant difference in the R:T ratios between the pacing sites (p < 0.001).
Conclusions: Pacing alters the R:T ratio significantly in most patients, theoretically increasing the risk for TWO and inappropriate shocks. Tailored programming for both devices is important for concomitant use of LPs and S-ICDs.
Keywords: Cardiac implantable devices; Leadless pacemakers; Personalised medicine; Subcutaneous implantable cardiac defibrillators.
© 2022. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The study was performed with ethical approval from Health Research Authority (HRA)—REC (20/NW/0366)—and was also granted local research and development (RHMCAR0528) approval. Consent to participate: All patients gave informed written consent prior to recruitment in the study. Conflict of interest: Dr. Mohamed ElRefai is receiving unrestricted grant from Boston Scientific. Dr. Paul Roberts receives consultancy fees from Boston Scientific and Medtronic. Prof. John Morgan is a senior medical director at Boston Scientific. Other authors have no conflicts of interest to declare.
Figures
References
-
- Randles DA, Hawkins NM, Shaw M, Patwala AY, Pettit SJ, Wright DJ. How many patients fulfil the surface electrocardiogram criteria for subcutaneous implantable cardioverter-defibrillator implantation? EP Europace. 2014;16(7):1015–21. 10.1093/EUROPACE/EUT370. - PubMed
-
- Olde Nordkamp LRA, Warnaars JLF, Kooiman KM, et al. Which patients are not suitable for a subcutaneous ICD: incidence and predictors of failed QRS-T-wave morphology screening. J Cardiovasc Electrophysiol. 2014;25(5):494–9. 10.1111/JCE.12343. - PubMed
-
- Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: an 8-year prospective multicenter study. Heart Rhythm. 2007;4(2):154–60. 10.1016/J.HRTHM.2006.10.009. - PubMed
-
- Udo EO, Zuithoff NPA, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012;9(5):728–35. 10.1016/J.HRTHM.2011.12.014. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

