Recent Advances in 1,2-Amino(hetero)arylation of Alkenes
- PMID: 35460596
- PMCID: PMC9357224
- DOI: 10.1002/asia.202200215
Recent Advances in 1,2-Amino(hetero)arylation of Alkenes
Abstract
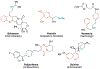
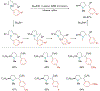
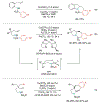
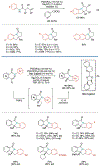
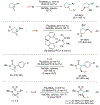
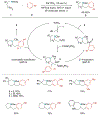
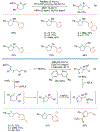
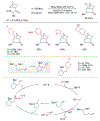
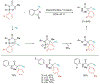
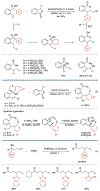
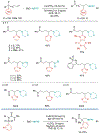
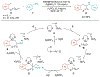
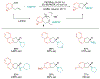
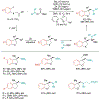
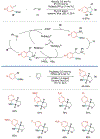
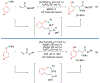
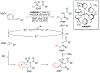
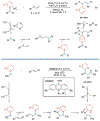
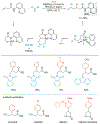
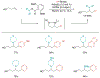
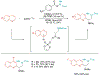
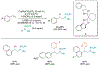
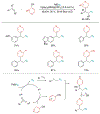
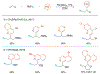
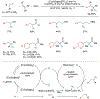
Alkene amino(hetero)arylation presents a highly efficient and straightforward strategy for direct installation of amino groups and heteroaryl rings across a double bond simultaneously. An extensive array of practical transformations has been developed via alkene difunctionalization approach to access a broad range of medicinally valuable (hetero)arylethylamine motifs. This review presents recent progress in 1,2-amino(hetero)arylation of alkenes organized in three different modes. First, intramolecular transformations employing C, N-tethered alkenes will be introduced. Next, two-component reactions will be discussed with different combination of precursors, N-tethered alkenes and external aryl precursor, C-tethered alkenes and external amine precursor, or C, N-tethered reagents, and alkenes. Last, three-component intermolecular amino(hetero)arylation reactions will be covered.
Keywords: (hetero)arylation; (hetero)arylethylamine; alkenes; amination; catalysis; difunctionalization.
© 2022 Wiley-VCH GmbH.
Figures
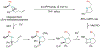

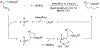
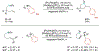
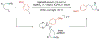
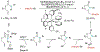
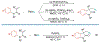

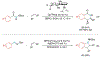

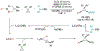

Similar articles
-
Design and Scalable Synthesis of N-Alkylhydroxylamine Reagents for the Direct Iron-Catalyzed Installation of Medicinally Relevant Amines*.Angew Chem Int Ed Engl. 2020 Nov 16;59(47):21064-21071. doi: 10.1002/anie.202008247. Epub 2020 Sep 15. Angew Chem Int Ed Engl. 2020. PMID: 32761827
-
A General Amino-(Hetero)arylation of Simple Olefins with (Hetero)aryl Sulfonamides Enabled by an N-Triazinyl Group.ACS Catal. 2025 Feb 7;15(3):2139-2149. doi: 10.1021/acscatal.5c00157. Epub 2025 Jan 22. ACS Catal. 2025. PMID: 40124959
-
1,2-Difunctionalization-type (hetero)arylation of unactivated alkenes triggered by radical addition/remote (hetero)aryl migration.Chem Commun (Camb). 2017 Apr 4;53(28):4038-4041. doi: 10.1039/c6cc09215b. Chem Commun (Camb). 2017. PMID: 28345103
-
Nitrogen-Centered Radicals in Functionalization of sp2 Systems: Generation, Reactivity, and Applications in Synthesis.Chem Rev. 2022 May 11;122(9):8181-8260. doi: 10.1021/acs.chemrev.1c00831. Epub 2022 Mar 14. Chem Rev. 2022. PMID: 35285636 Review.
-
Difunctionalization of Alkenes and Alkynes via Intermolecular Radical and Nucleophilic Additions.Molecules. 2020 Dec 28;26(1):105. doi: 10.3390/molecules26010105. Molecules. 2020. PMID: 33379397 Free PMC article. Review.
Cited by
-
Arylthianthrenium Salts for Triplet Energy Transfer Catalysis.J Am Chem Soc. 2024 Nov 6;146(44):30474-30482. doi: 10.1021/jacs.4c11099. Epub 2024 Oct 28. J Am Chem Soc. 2024. PMID: 39466322 Free PMC article.
-
Photoinduced Copper-Catalyzed Late-Stage Azidoarylation of Alkenes via Arylthianthrenium Salts.J Am Chem Soc. 2023 Jun 28;145(25):13542-13548. doi: 10.1021/jacs.3c04016. Epub 2023 Jun 12. J Am Chem Soc. 2023. PMID: 37307146 Free PMC article.
-
Regioselective intermolecular carboamination of allylamines via nucleopalladation: empowering three-component synthesis of vicinal diamines.Chem Sci. 2024 Nov 29;16(1):386-392. doi: 10.1039/d4sc07630c. eCollection 2024 Dec 18. Chem Sci. 2024. PMID: 39620079 Free PMC article.
-
Chiral arylsulfinylamides as reagents for visible light-mediated asymmetric alkene aminoarylations.Nat Chem. 2024 Apr;16(4):607-614. doi: 10.1038/s41557-023-01414-8. Epub 2024 Jan 16. Nat Chem. 2024. PMID: 38228849 Free PMC article.
-
Harnessing Bromo/Acyloxy Transposition (BrAcT) and Excited-State Copper Catalysis for Styrene Difunctionalization.J Am Chem Soc. 2024 Aug 7;146(31):21271-21279. doi: 10.1021/jacs.4c08984. Epub 2024 Jul 23. J Am Chem Soc. 2024. PMID: 39042434 Free PMC article.
References
-
- Zeng X, Chem. Rev 2013, 113, 6864–6900. - PubMed
-
- Njardarson Group. Top Pharmaceuticals Poster. njardarson.lab.arizona.edu/content/top-pharmaceuticals-poster (accessed 2020)
-
- Aube J, Peng X, Wang Y, Takusagawa F, J. Am. Chem. Soc 1992, 114, 5466–5467.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

