Sentinel lymph node procedure in patients with recurrent vulvar squamous cell carcinoma: a proposed protocol for a multicentre observational study
- PMID: 35461213
- PMCID: PMC9034534
- DOI: 10.1186/s12885-022-09543-y
Sentinel lymph node procedure in patients with recurrent vulvar squamous cell carcinoma: a proposed protocol for a multicentre observational study
Abstract
Background: Standard groin treatment in recurrent vulvar cancer consists of uni- or bilateral inguinofemoral lymphadenectomy (IFL), whereas in the primary setting women with selected unifocal tumours will undergo a sentinel lymph node (SLN) procedure. The SLN procedure results in fewer short and long-term sequelae compared to IFL, but some concerns must first be considered. Lymph drainage of the vulvar region can be affected by a previous surgery, which might reduce the number of detectable SLN nodes (feasibility) but increase the chance of encountering aberrant lymph drainage patterns such as bilateral SLNs in lateral tumours or SLNs at unexpected locations. Therefore, the SLN procedure potentially carries a higher risk of groin recurrence if a tumour positive node is not retrieved, but may also improve outcomes for women with aberrant drainage patterns. Since the relative benefits and drawbacks of the SLN procedure are still unclear we will investigate the safety of the SLN procedure in women with a first recurrent vulvar cancer. In a simultaneously started registration study we prospectively gather information on women with a first recurrence of vulvar cancer ineligible for the SLN procedure.
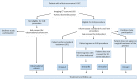
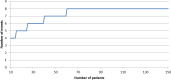
Method: In this prospective multicentre observational study all women with a first recurrence of vulvar cancer will be asked to consent to the collection of information on their diagnostics, treatment and outcome, and to complete quality of life and lymph oedema questionnaires. Women with unifocal tumours smaller than 4 cm and unsuspicious groin nodes will be offered the SLN procedure, with follow-up every 3 months together with imaging at 6 and 12 months when the SLN is tumour negative. The primary outcome is groin recurrence within 2 years of initial surgery. A total of 150 women with negative SLNs will be required to demonstrate safety, a stopping rule will apply and an extensive statistical analysis has been designed.
Discussion: Should the SLN procedure prove feasible and safe in recurrent vulvar cancer, it will be available for implementation in clinics worldwide. The inclusion of women ineligible for the SLN procedure in the current prospective study will help to bridge knowledge gaps and define future research questions.
Trial registration: Medical Ethical Committee approval number NL70149.078.19 (trial protocol version 2.0, date March 2nd, 2020). Affiliation: Erasmus Medical Centre. Dutch trial register NL8467 . Date of registration 19.03.2020.
Keywords: Recurrence; Sentinel lymph node; Vulvar cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Sentinel lymph node procedure in early-stage vulvar cancer: Correlation of lymphoscintigraphy with surgical outcome and groin recurrence.Eur J Surg Oncol. 2023 Oct;49(10):107006. doi: 10.1016/j.ejso.2023.107006. Epub 2023 Aug 3. Eur J Surg Oncol. 2023. PMID: 37572588
-
Long-term oncological outcomes of patients with negative sentinel lymph node in vulvar cancer. Comparative study with conventional lymphadenectomy.Acta Obstet Gynecol Scand. 2018 Dec;97(12):1427-1437. doi: 10.1111/aogs.13431. Epub 2018 Aug 26. Acta Obstet Gynecol Scand. 2018. PMID: 30063814
-
Phase II activity trial of high-dose radiation and chemosensitization in patients with macrometastatic lymph node spread after sentinel node biopsy in vulvar cancer: GROningen INternational Study on Sentinel nodes in Vulvar cancer III (GROINSS-V III/NRG-GY024).Int J Gynecol Cancer. 2023 Apr 3;33(4):619-622. doi: 10.1136/ijgc-2022-004122. Int J Gynecol Cancer. 2023. PMID: 36653060 Free PMC article. Clinical Trial.
-
Postoperative management of vulvar cancer.Int J Gynecol Cancer. 2022 Mar;32(3):338-343. doi: 10.1136/ijgc-2021-002463. Int J Gynecol Cancer. 2022. PMID: 35256421 Review.
-
Update on the sentinel lymph node procedure in vulvar cancer.Expert Rev Anticancer Ther. 2010 Jan;10(1):61-9. doi: 10.1586/era.09.125. Expert Rev Anticancer Ther. 2010. PMID: 20014886 Review.
Cited by
-
Time to extend the indication for sentinel node biopsy in vulvar cancer? Results from a prospective nationwide Swedish study.Int J Gynecol Cancer. 2023 Dec 4;33(12):1845-1852. doi: 10.1136/ijgc-2023-004790. Int J Gynecol Cancer. 2023. PMID: 37918956 Free PMC article.
-
Incidence of inguinofemoral lymph node metastases at the first local recurrence of vulvar cancer: a Dutch nationwide study.Br J Cancer. 2023 Oct;129(6):956-964. doi: 10.1038/s41416-023-02373-0. Epub 2023 Jul 28. Br J Cancer. 2023. PMID: 37507545 Free PMC article.
-
Current Limitations of Sentinel Node Biopsy in Vulvar Cancer.Curr Oncol. 2025 Apr 8;32(4):215. doi: 10.3390/curroncol32040215. Curr Oncol. 2025. PMID: 40277771 Free PMC article. Review.
-
European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer - Update 2023.Int J Gynecol Cancer. 2023 Jul 3;33(7):1023-1043. doi: 10.1136/ijgc-2023-004486. Int J Gynecol Cancer. 2023. PMID: 37369376 Free PMC article.
-
Comparison of two hybrid sentinel node tracers: indocyanine green (ICG)-99mTc-nanocolloid vs. ICG-99mTc-nanoscan from a nuclear medicine and surgical perspective.Eur J Nucl Med Mol Imaging. 2023 Jul;50(8):2282-2291. doi: 10.1007/s00259-023-06157-9. Epub 2023 Mar 17. Eur J Nucl Med Mol Imaging. 2023. PMID: 36929210 Free PMC article.
References
-
- Coleman RL, Ali S, Levenback CF, Gold MA, Fowler JM, Judson PL, et al. Is bilateral lymphadenectomy for midline squamous carcinoma of the vulva always necessary? An analysis from gynecologic oncology group (GOG) 173. Gynecol Oncol. 2013;128(2):155–159. doi: 10.1016/j.ygyno.2012.11.034. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

