Autophagy guards tendon homeostasis
- PMID: 35461310
- PMCID: PMC9035152
- DOI: 10.1038/s41419-022-04824-7
Autophagy guards tendon homeostasis
Abstract
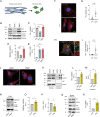
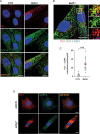
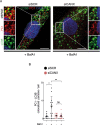
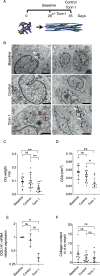
Tendons are vital collagen-dense specialized connective tissues transducing the force from skeletal muscle to the bone, thus enabling movement of the human body. Tendon cells adjust matrix turnover in response to physiological tissue loading and pathological overloading (tendinopathy). Nevertheless, the regulation of tendon matrix quality control is still poorly understood and the pathogenesis of tendinopathy is presently unsolved. Autophagy, the major mechanism of degradation and recycling of cellular components, plays a fundamental role in the homeostasis of several tissues. Here, we investigate the contribution of autophagy to human tendons' physiology, and we provide in vivo evidence that it is an active process in human tendon tissue. We show that selective autophagy of the endoplasmic reticulum (ER-phagy), regulates the secretion of type I procollagen (PC1), the major component of tendon extracellular matrix. Pharmacological activation of autophagy by inhibition of mTOR pathway alters the ultrastructural morphology of three-dimensional tissue-engineered tendons, shifting collagen fibrils size distribution. Moreover, autophagy induction negatively affects the biomechanical properties of the tissue-engineered tendons, causing a reduction in mechanical strength under tensile force. Overall, our results provide the first evidence that autophagy regulates tendon homeostasis by controlling PC1 quality control, thus potentially playing a role in the development of injured tendons.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

