A superior loading control for the cellular thermal shift assay
- PMID: 35461337
- PMCID: PMC9035151
- DOI: 10.1038/s41598-022-10653-7
A superior loading control for the cellular thermal shift assay
Abstract
The cellular thermal shift assay (CETSA), as a method to determine protein-ligand interaction and cellular protein modification, has rapidly become routine laboratory practice. However, current options to determine that (1) sample was loaded in each lane of the analysed western blot and (2) the amount loaded was equal, are suboptimal. Here, we report that the αC-terminal fragment of the amyloid precursor protein (APP-αCTF), detected in several wild-type mammalian cell lines, is a highly stable, soluble protein equally present from 4 to 95 °C. We demonstrate that the level of traditional loading controls (vinculin, GAPDH, β-actin, heat-shock chaperone 70 and superoxide dismutase-1) are all temperature sensitive. Additionally, both APP-CTFs (α and β) behaved similarly upon temperature exposure while APP-βCTF levels were not influenced by the presence of a binding ligand either. This emphasises that these proteins can be used as a loading control in the unlikely event of off-target binding during ligand screening. A working example is also presented for mitogen-activated protein kinase kinase in the presence of two inhibitors, PD184352 and U0126, where APP-αCTF was used to normalise the data across experimental replicates. A reduction in data variance and standard deviations was observed after normalisation. Conclusively, APP-αCTF is a superior CETSA loading control that can be used as a standard for this technique.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
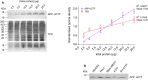


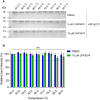

 ]) (C), calculated by setting the highest intensity for each data set as 100% and the curve generated using the Boltzmann sigmoidal equation are shown with (D) the mean aggregation temperature (Tagg) ± SD, pre-normalised (open symbol) vs normalised (N—solid symbol) (n = 3). One-way ANOVA with Tukey’s post-hoc was performed where ns, not significant; **p ≤ 0.01; ****p ≤ 0.0001. See supplementary information file, raw data images for full-length western blots.
]) (C), calculated by setting the highest intensity for each data set as 100% and the curve generated using the Boltzmann sigmoidal equation are shown with (D) the mean aggregation temperature (Tagg) ± SD, pre-normalised (open symbol) vs normalised (N—solid symbol) (n = 3). One-way ANOVA with Tukey’s post-hoc was performed where ns, not significant; **p ≤ 0.01; ****p ≤ 0.0001. See supplementary information file, raw data images for full-length western blots.References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

