The performance of wearable sensors in the detection of SARS-CoV-2 infection: a systematic review
- PMID: 35461692
- PMCID: PMC9020803
- DOI: 10.1016/S2589-7500(22)00019-X
The performance of wearable sensors in the detection of SARS-CoV-2 infection: a systematic review
Abstract
Containing the COVID-19 pandemic requires rapidly identifying infected individuals. Subtle changes in physiological parameters (such as heart rate, respiratory rate, and skin temperature), discernible by wearable devices, could act as early digital biomarkers of infections. Our primary objective was to assess the performance of statistical and algorithmic models using data from wearable devices to detect deviations compatible with a SARS-CoV-2 infection. We searched MEDLINE, Embase, Web of Science, the Cochrane Central Register of Controlled Trials (known as CENTRAL), International Clinical Trials Registry Platform, and ClinicalTrials.gov on July 27, 2021 for publications, preprints, and study protocols describing the use of wearable devices to identify a SARS-CoV-2 infection. Of 3196 records identified and screened, 12 articles and 12 study protocols were analysed. Most included articles had a moderate risk of bias, as per the National Institute of Health Quality Assessment Tool for Observational and Cross-Sectional Studies. The accuracy of algorithmic models to detect SARS-CoV-2 infection varied greatly (area under the curve 0·52-0·92). An algorithm's ability to detect presymptomatic infection varied greatly (from 20% to 88% of cases), from 14 days to 1 day before symptom onset. Increased heart rate was most frequently associated with SARS-CoV-2 infection, along with increased skin temperature and respiratory rate. All 12 protocols described prospective studies that had yet to be completed or to publish their results, including two randomised controlled trials. The evidence surrounding wearable devices in the early detection of SARS-CoV-2 infection is still in an early stage, with a limited overall number of studies identified. However, these studies show promise for the early detection of SARS-CoV-2 infection. Large prospective, and preferably controlled, studies recruiting and retaining larger and more diverse populations are needed to provide further evidence.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests MM, BMG, VK, BF, DV, DEG, MC, and GSD received grants from Innovative Medicines Initiative 2 Joint Undertaking (number 101005177), during the conduct of the study. BMG reports consulting fees and employment from Ava Science, support for attending meetings and travel from Ava Aktiengesellschaft (AG), a patent application from Ava AG (P24892CH00) filed with the Swiss Federal Institute of Intellectual Property for System and Method for Pre-Symptomatic and/or Asymptomatic Detection of a Human Viral or Bacterial Infection based on pilot data from the COVID-RED clinical study, and consultancy for Falcon Health and TheraB Medical, outside the submitted work. VK reports employment from Ava Science and Ava AG, during the conduct of this study. TBB, BF, DV, and DEG report employment from Julius Clinical Research, during the conduct of the study. MC reports employment from Ava AG during the conduct of the study. GSD reports a grant from Health Holland, outside the submitted work. All other authors declare no competing interests.
Figures
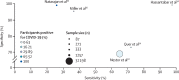
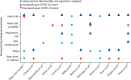
References
-
- WHO Novel coronavirus (2019-nCoV) situation report-1. Jan 21, 2020. https://apps.who.int/iris/bitstream/handle/10665/330760/nCoVsitrep21Jan2...
-
- WHO WHO coronavirus (COVID-19) dashboard. 2020. https://covid19.who.int/
-
- WHO Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance. March 19, 2020. https://apps.who.int/iris/handle/10665/331501
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

