Sulforaphane induces lipophagy through the activation of AMPK-mTOR-ULK1 pathway signaling in adipocytes
- PMID: 35461903
- PMCID: PMC9447841
- DOI: 10.1016/j.jnutbio.2022.109017
Sulforaphane induces lipophagy through the activation of AMPK-mTOR-ULK1 pathway signaling in adipocytes
Abstract
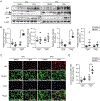
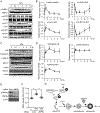
Lipophagy, a form of selective autophagy, degrades lipid droplet (LD) in adipose tissue and the liver. The chemotherapeutic isothiocyanate sulforaphane (SFN) contributes to lipolysis through the activation of hormone-sensitive lipase and the browning of white adipocytes. However, the details concerning the regulation of lipolysis in adipocytes by SFN-mediated autophagy remain unclear. In this study, we investigated the effects of SFN on autophagy in the epididymal fat of mice fed a high-fat diet (HFD) or control-fat diet and on the molecular mechanisms of autophagy in differentiated 3T3-L1 cells. Western blotting revealed that the protein expression of lipidated LC3 (LC3-II), an autophagic substrate, was induced after 3T3-L1 adipocytes treatment with SFN. In addition, SFN increased the LC3-II protein expression in the epididymal fat of mice fed an HFD. Immunofluorescence showed that the SFN-induced LC3 expression was co-localized with LDs in 3T3-L1 adipocytes and with perilipin, the most abundant adipocyte-specific protein, in adipocytes of mice fed an HFD. Next, we confirmed that SFN activates autophagy flux in differentiated 3T3-L1 cells using the mCherry-EGFP-LC3 and GFP-LC3-RFP-LC3ΔG probe. Furthermore, we examined the induction mechanisms of autophagy by SFN in 3T3-L1 adipocytes using western blotting. ATG5 knockdown partially blocked the SFN-induced release of fatty acids from LDs in mature 3T3-L1 adipocytes. SFN time-dependently elicited the phosphorylation of AMPK, the dephosphorylation of mTOR, and the phosphorylation of ULK1 in differentiated 3T3-L1 cells. Taken together, these results suggest that SFN may provoke lipophagy through AMPK-mTOR-ULK1 pathway signaling, resulting in partial lipolysis of adipocytes.
Keywords: Adipose; Autophagy; Cell biology; Dietary factors; Lipid droplets; Obesity.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interests
The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Kopelman PG. Obesity as a medical problem. Nature 2000;404:635–43. - PubMed
-
- Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017;376:254–66. - PubMed
-
- Bray GA. A concise review on the therapeutics of obesity. Nutrition 2000;16:953–60. - PubMed
-
- Abuduli M, Ohminami H, Otani T, Kubo H, Ueda H, Kawai Y, et al. Effects of dietary phosphate on glucose and lipid metabolism. Am J Physiol Endocrinol Metab 2016;310:E526–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

