Development of a Hemostatic Urinary Catheter for Transurethral Prostatic Surgical Applications
- PMID: 35461919
- PMCID: PMC10860670
- DOI: 10.1016/j.urology.2022.03.037
Development of a Hemostatic Urinary Catheter for Transurethral Prostatic Surgical Applications
Abstract
Objective: To investigate a novel transurethral hemostatic catheter device with an integrated chitosan endoluminal hemostatic dressing (CEHD). Development and implementation of this technology may help address bleeding following surgery such as transurethral resection of prostate (TURP). Bleeding remains the most common complication following TURP, leading to increased morbidity and hospitalization.
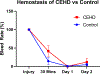
Methods: Investigation of hemostasis, delivery, safety and efficacy of the CEHD device is conducted using Female Yorkshire swine (N = 23). Hemostatic efficacy of the CEHD (N = 12) is investigated against a control of gauze (N = 12) in a splenic injury model (3 swine). The delivery, safety, and efficacy of the CEHD device (N = 10) are investigated against Foley-catheter control (N = 10) for 7 days using a swine bladder-neck-injury model.
Results: In the splenic injury study, 9/12 CEHD dressings successfully achieved hemostasis within 150 seconds (mean 83 seconds) vs success of 6/12 (mean 150 seconds) for gauze (P = .04). In the 7-day study, the CEHD was successfully deployed in 10/10 animals and all dressings were tolerated without histologic or clinical adverse effect. Hemostasis of the CEHD device was found to be noninferior to control catheters. Noninferiority is attributed to low bleeding rates in the swine bladder neck injury model.
Conclusion: This investigation successfully demonstrated the feasibility of transurethral deployment of the CEHD in vivo. Routine use of safe and slowly dissolvable CEHDs could reduce the rate of complications and hospitalizations associated with bleeding and blood loss in TURP procedures. Further investigation is warranted.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Benign Prostatic Hyperplasia.J Urol. 2022 Dec;208(6):1323-1325. doi: 10.1097/JU.0000000000002948. Epub 2022 Sep 1. J Urol. 2022. PMID: 36047282 No abstract available.
Similar articles
-
The effect of fibrinogen concentrate on perioperative bleeding in transurethral resection of the prostate: a double-blind placebo-controlled and randomized study.J Thromb Haemost. 2017 Feb;15(2):255-262. doi: 10.1111/jth.13575. Epub 2017 Feb 1. J Thromb Haemost. 2017. PMID: 27888575 Clinical Trial.
-
A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams).J Urol. 2003 Oct;170(4 Pt 1):1270-4. doi: 10.1097/01.ju.0000086948.55973.00. J Urol. 2003. PMID: 14501739 Clinical Trial.
-
Safer transurethral resection of the prostate: coagulating intermittent cutting reduces hemostatic complications.J Urol. 2004 Jan;171(1):289-91. doi: 10.1097/01.ju.0000098925.76817.3a. J Urol. 2004. PMID: 14665896
-
Minimally invasive surgery in the management of benign prostatic hyperplasia.Minerva Urol Nefrol. 2009 Sep;61(3):269-89. Minerva Urol Nefrol. 2009. PMID: 19773728 Review.
-
Electrosurgical transurethral resection of the prostate and transurethral incision of the prostate (monopolar techniques).Can J Urol. 2015 Oct;22 Suppl 1:24-9. Can J Urol. 2015. PMID: 26497341 Review.
References
-
- FF EB. A hemostatic bag catheter. J Urol. 1937;38:134–139. 10.1016/S0022-5347(17)71935-0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

