Moment of truth-adding carboplatin to neoadjuvant/adjuvant chemotherapy in triple negative breast cancer improves overall survival: An individual participant data and trial-level Meta-analysis
- PMID: 35462344
- PMCID: PMC9039877
- DOI: 10.1016/j.breast.2022.04.006
Moment of truth-adding carboplatin to neoadjuvant/adjuvant chemotherapy in triple negative breast cancer improves overall survival: An individual participant data and trial-level Meta-analysis
Abstract
Importance: Carboplatin increases the pathological complete remission (pCR) rate in triple negative breast cancer (TNBC) when added to neoadjuvant chemotherapy, however, evidence on its effect on survival outcomes is controversial.
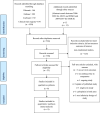
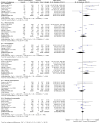
Methods: The study was prospectively registered at PROSPERO (CRD42021228386). We systematically searched PubMed, Embase, Cochrane Central Register of Clinical Trials, and conference proceedings from January 1, 2004 to January 30, 2022 for relevant randomized clinical trials (RCTs) of (neo)adjuvant chemotherapy in TNBC patients, with carboplatin in the intervention arm and standard anthracycline taxane (AT) in the control arm. PRISMA guidelines were used for this review. Data were pooled using fixed and random effects models as appropriate on extracted hazard ratios (HR). Individual patient data (IPD)for disease free survival (DFS) and overall survival (OS) were extracted from published survival curves of included RCTs; DFS and OS curves for each trial and the combined population were reconstructed, and HR estimated. The primary outcome was DFS; OS, pCR, and toxicity were secondary outcomes.
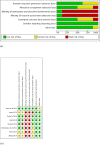
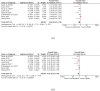
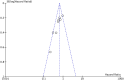
Results: Eight trials with 2425 patients were included. Carboplatin improved DFS (HR 0.60; 95% CI 0.47 to 0.78; I2 45%, p < 0.001) compared with AT at trial level and IPD level (HR 0.66; 95%CI, 0.55 to 0.80, p < 0.001) analysis. The OS also improved with carboplatin at both trial level (HR 0.69, 95%CI 0.50 to 0.95, I2 41%, p = 0.02) and IPD level (HR 0.68; 95%CI, 0.54 to 0.87, p = 0.002) analysis. The pCR as expected, was better in the carboplatin arm (OR 2.11; 95% CI = 1.44-3.08; I2 67%, p = 0.009). Anaemia and thrombocytopaenia were higher in the carboplatin arm.
Conclusion: and relevance: Carboplatin added to (neo)adjuvant chemotherapy in TNBC improves survival, as shown in both trial level and IPD analysis.
Keywords: Adjuvant; Carboplatin; Chemotherapy; Neoadjuvant; Platinum; TNBC; Triple negative breast cancer.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





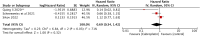
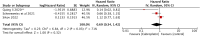

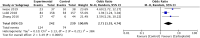
References
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

