S-Nitrosylation of cathepsin B affects autophagic flux and accumulation of protein aggregates in neurodegenerative disorders
- PMID: 35462559
- PMCID: PMC9613756
- DOI: 10.1038/s41418-022-01004-0
S-Nitrosylation of cathepsin B affects autophagic flux and accumulation of protein aggregates in neurodegenerative disorders
Abstract
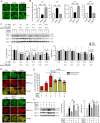
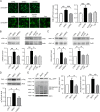
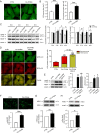
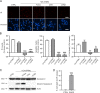
Protein S-nitrosylation is known to regulate enzymatic function. Here, we report that nitric oxide (NO)-related species can contribute to Alzheimer's disease (AD) by S-nitrosylating the lysosomal protease cathepsin B (forming SNO-CTSB), thereby inhibiting CTSB activity. This posttranslational modification inhibited autophagic flux, increased autolysosomal vesicles, and led to accumulation of protein aggregates. CA-074Me, a CTSB chemical inhibitor, also inhibited autophagic flux and resulted in accumulation of protein aggregates similar to the effect of SNO-CTSB. Inhibition of CTSB activity also induced caspase-dependent neuronal apoptosis in mouse cerebrocortical cultures. To examine which cysteine residue(s) in CTSB are S-nitrosylated, we mutated candidate cysteines and found that three cysteines were susceptible to S-nitrosylation. Finally, we observed an increase in SNO-CTSB in both 5XFAD transgenic mouse and flash-frozen postmortem human AD brains. These results suggest that S-nitrosylation of CTSB inhibits enzymatic activity, blocks autophagic flux, and thus contributes to AD pathogenesis.
© 2022. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare that YHK is a shareholder of Zincure Corp., and that KRK is currently employed by Zincure Corp. SAL is a scientific founder of Adamas Pharmaceuticals, Inc., EuMentis Therapeutics, Inc., and Infla
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

