Tofacitinib and newer JAK inhibitors in inflammatory bowel disease-where we are and where we are going
- PMID: 35462642
- PMCID: PMC9007061
- DOI: 10.7573/dic.2021-11-4
Tofacitinib and newer JAK inhibitors in inflammatory bowel disease-where we are and where we are going
Abstract
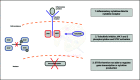
Inflammatory bowel diseases, comprising ulcerative colitis (UC) and Crohn's disease, are chronic, immune-mediated and progressive inflammatory disorders affecting the gastrointestinal tract. Tofacitinib is the first oral small-molecule Janus kinase (JAK) inhibitor licensed and approved by the National Institute for Health and Care Excellence (NICE) for use in moderately-to-severely active UC after intolerance, inadequate response, or loss of response to conventional treatment or biologic therapy. The pivotal OCTAVE studies demonstrated the efficacy and safety of tofacitinib for the induction and maintenance of remission in UC. A growing body of evidence from real-world data supports the positive clinical and endoscopic benefits observed with tofacitinib treatment in the OCTAVE trials. This narrative review summarizes the current literature regarding the mechanism of action of tofacitinib, data from registrational trials, emerging real-world evidence, and an overview of the most recent safety evidence. We explore evolving treatment paradigms, including the use of tofacitinib in the COVID-19 era, pregnancy and extraintestinal manifestations, as well as the emerging concept of combining tofacitinib with biological therapy. We will also present a brief overview of the next generation of JAK inhibitors in the pipeline.
Keywords: Crohn’s disease; Janus kinase inhibitors; effectiveness; efficacy; inflammatory bowel diseases; safety; tofacitinib; ulcerative colitis.
Copyright © 2022 Liu E, Aslam N, Nigam G, Limdi JK.
Conflict of interest statement
Disclosure and potential conflicts of interest: EL and JKL have a non-pharmaceutical investigator-initiated sponsored research grant from Galapagos. EL has received speaker fees from Janssen. JKL has received speaker and consultancy fees from Abbvie, Arena, MSD, Galapagos, Janssen, Takeda, Pfizer and Tillots, and research support from Galapagos and Takeda. EL has received speaker fees from Janssen. The authors declare that they have no other conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/03/dic.2021-11-4-COI.pdf
Figures
References
-
- Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45(10):1291–1302. doi: 10.1111/apt.14030. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources