Therapeutic Devices for Motor Symptoms in Parkinson's Disease: Current Progress and a Systematic Review of Recent Randomized Controlled Trials
- PMID: 35462692
- PMCID: PMC9020378
- DOI: 10.3389/fnagi.2022.807909
Therapeutic Devices for Motor Symptoms in Parkinson's Disease: Current Progress and a Systematic Review of Recent Randomized Controlled Trials
Abstract
Background: Pharmacotherapy is the first-line treatment option for Parkinson's disease, and levodopa is considered the most effective drug for managing motor symptoms. However, side effects such as motor fluctuation and dyskinesia have been associated with levodopa treatment. For these conditions, alternative therapies, including invasive and non-invasive medical devices, may be helpful. This review sheds light on current progress in the development of devices to alleviate motor symptoms in Parkinson's disease.
Methods: We first conducted a narrative literature review to obtain an overview of current invasive and non-invasive medical devices and thereafter performed a systematic review of recent randomized controlled trials (RCTs) of these devices.
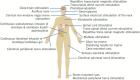
Results: Our review revealed different characteristics of each device and their effectiveness for motor symptoms. Although invasive medical devices are usually highly effective, surgical procedures can be burdensome for patients and have serious side effects. In contrast, non-pharmacological/non-surgical devices have fewer complications. RCTs of non-invasive devices, especially non-invasive brain stimulation and mechanical peripheral stimulation devices, have proven effectiveness on motor symptoms. Nearly no non-invasive devices have yet received Food and Drug Administration certification or a CE mark.
Conclusion: Invasive and non-invasive medical devices have unique characteristics, and several RCTs have been conducted for each device. Invasive devices are more effective, while non-invasive devices are less effective and have lower hurdles and risks. It is important to understand the characteristics of each device and capitalize on these.
Keywords: Parkinson’s disease; freezing of gait (FOG); gait; invasive medical devices; non-invasive medical device; stimulation; tremor.
Copyright © 2022 Fujikawa, Morigaki, Yamamoto, Oda, Nakanishi, Izumi and Takagi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

