Decreased Electroencephalography Global Field Synchronization in Slow-Frequency Bands Characterizes Synaptic Dysfunction in Amnestic Subtypes of Mild Cognitive Impairment
- PMID: 35462693
- PMCID: PMC9031731
- DOI: 10.3389/fnagi.2022.755454
Decreased Electroencephalography Global Field Synchronization in Slow-Frequency Bands Characterizes Synaptic Dysfunction in Amnestic Subtypes of Mild Cognitive Impairment
Abstract
Background: Mild cognitive impairment (MCI) is highly prevalent in a memory clinic setting and is heterogeneous regarding its clinical presentation, underlying pathophysiology, and prognosis. The most prevalent subtypes are single-domain amnestic MCI (sd-aMCI), considered to be a prodromal phase of Alzheimer's disease (AD), and multidomain amnestic MCI (md-aMCI), which is associated with multiple etiologies. Since synaptic loss and dysfunction are the closest pathoanatomical correlates of AD-related cognitive impairment, we aimed to characterize it in patients with sd-aMCI and md-aMCI by means of resting-state electroencephalography (EEG) global field power (GFP), global field synchronization (GFS), and novel cerebrospinal fluid (CSF) synaptic biomarkers.
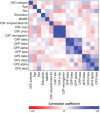
Methods: We included 52 patients with sd-aMCI (66.9 ± 7.3 years, 52% women) and 30 with md-aMCI (63.1 ± 7.1 years, 53% women). All patients underwent a detailed clinical assessment, resting-state EEG recordings and quantitative analysis (GFP and GFS in delta, theta, alpha, and beta bands), and analysis of CSF biomarkers of synaptic dysfunction, neurodegeneration, and AD-related pathology. Cognitive subtyping was based on a comprehensive neuropsychological examination. The Mini-Mental State Examination (MMSE) was used as an estimation of global cognitive performance. EEG and CSF biomarkers were included in a multivariate model together with MMSE and demographic variables, to investigate differences between sd-aMCI and md-aMCI.
Results: Patients with sd-aMCI had higher CSF phosphorylated tau, total tau and neurogranin levels, and lower values in GFS delta and theta. No differences were observed in GFP. The multivariate model showed that the most important synaptic measures for group separation were GFS theta, followed by GFS delta, GFP theta, CSF neurogranin, and GFP beta.
Conclusion: Patients with sd-aMCI when compared with those with md-aMCI have a neurophysiological and biochemical profile of synaptic damage, neurodegeneration, and amyloid pathology closer to that described in patients with AD. The most prominent signature in sd-aMCI was a decreased global synchronization in slow-frequency bands indicating that functional connectivity in slow frequencies is more specifically related to early effects of AD-specific molecular pathology.
Keywords: Alzheimer’s disease; EEG power; amnestic mild cognitive impairment (aMCI); electroencephalography; global field synchronization (GFS); synaptic dysfunction.
Copyright © 2022 Smailovic, Ferreira, Ausén, Ashton, Koenig, Zetterberg, Blennow and Jelic.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The role of quantitative EEG biomarkers in Alzheimer's disease and mild cognitive impairment: applications and insights.Front Aging Neurosci. 2025 Apr 23;17:1522552. doi: 10.3389/fnagi.2025.1522552. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40336944 Free PMC article. Review.
-
Synaptic Molecular and Neurophysiological Markers Are Independent Predictors of Progression in Alzheimer's Disease.J Alzheimers Dis. 2021;83(1):355-366. doi: 10.3233/JAD-201234. J Alzheimers Dis. 2021. PMID: 34334389 Free PMC article.
-
Quantitative EEG power and synchronization correlate with Alzheimer's disease CSF biomarkers.Neurobiol Aging. 2018 Mar;63:88-95. doi: 10.1016/j.neurobiolaging.2017.11.005. Epub 2017 Nov 16. Neurobiol Aging. 2018. PMID: 29245058
-
Alteration of regional homogeneity and white matter hyperintensities in amnestic mild cognitive impairment subtypes are related to cognition and CSF biomarkers.Brain Imaging Behav. 2018 Feb;12(1):188-200. doi: 10.1007/s11682-017-9680-4. Brain Imaging Behav. 2018. PMID: 28236166
-
Neuropsychological and neuroimaging characteristics of amnestic mild cognitive impairment subtypes: a selective overview.CNS Neurosci Ther. 2015 Oct;21(10):776-83. doi: 10.1111/cns.12391. Epub 2015 Mar 26. CNS Neurosci Ther. 2015. PMID: 25809732 Free PMC article. Review.
Cited by
-
Electrocorticographic Activation Patterns of Electroencephalographic Microstates.Brain Topogr. 2024 Mar;37(2):287-295. doi: 10.1007/s10548-023-00952-1. Epub 2023 Mar 20. Brain Topogr. 2024. PMID: 36939988 Free PMC article.
-
Time-Frequency Domain Analysis of Quantitative Electroencephalography as a Biomarker for Dementia.Diagnostics (Basel). 2025 Jun 13;15(12):1509. doi: 10.3390/diagnostics15121509. Diagnostics (Basel). 2025. PMID: 40564830 Free PMC article. Review.
-
The role of quantitative EEG biomarkers in Alzheimer's disease and mild cognitive impairment: applications and insights.Front Aging Neurosci. 2025 Apr 23;17:1522552. doi: 10.3389/fnagi.2025.1522552. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40336944 Free PMC article. Review.
-
Quantitative electroencephalography (qEEG), apolipoprotein A-I (APOA-I), and apolipoprotein epsilon 4 (APOE ɛ4) alleles for the diagnosis of mild cognitive impairment and Alzheimer's disease.Neurol Sci. 2024 Feb;45(2):547-556. doi: 10.1007/s10072-023-07028-9. Epub 2023 Sep 6. Neurol Sci. 2024. PMID: 37673807
-
An EEG-based framework for automated discrimination of conversion to Alzheimer's disease in patients with amnestic mild cognitive impairment: an 18-month longitudinal study.Front Aging Neurosci. 2025 Jan 6;16:1470836. doi: 10.3389/fnagi.2024.1470836. eCollection 2024. Front Aging Neurosci. 2025. PMID: 39834619 Free PMC article.
References
-
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7 270–279. 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
-
- Amit Y., Geman D. (1997). Shape quantization and recognition with randomized trees. Neural Comput. 9 1545–1588. 10.1162/neco.1997.9.7.1545 - DOI
-
- Bogdanovic N. D., Gottfries J., Volkman I., Winblad B., Blennow K. (2002). Regional and Cellular Distribution of Synaptic Proteins in the Normal Human Brain. Brain Aging 2 18–30.
LinkOut - more resources
Full Text Sources

