High precision-cut liver slice model to study cell-autonomous antiviral defense of hepatocytes within their microenvironment
- PMID: 35462860
- PMCID: PMC9019249
- DOI: 10.1016/j.jhepr.2022.100465
High precision-cut liver slice model to study cell-autonomous antiviral defense of hepatocytes within their microenvironment
Abstract
Background & aims: Increased sensitivity towards tumor necrosis factor (TNF)-induced cell death in virus-infected hepatocytes has revealed a so far unrecognized hepatocyte-intrinsic antiviral immune surveillance mechanism, for which no in vitro or ex vivo model is available. We aimed to establish precision-cut liver slices (PCLS) as a model system to study hepatocyte-intrinsic regulation of apoptosis.
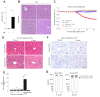
Methods: Preparation of PCLS from mouse and human liver tissue was optimized for minimal procedure-associated apoptosis. Functionality of liver cells in PCLS was characterized using extracellular flux analysis to determine mitochondrial respiration, and viral infection with recombinant adenovirus and lymphocytic choriomeningitis virus (LCMV) was used to probe for hepatocyte-intrinsic sensitivity towards apoptosis in PCLS. Apoptosis was detected by immunohistochemical staining for cleaved-caspase 3 and quantified by detection of effector caspase activity in PCLS.
Results: We established an optimized protocol for preparation of PCLS from human and mouse models using agarose-embedding of liver tissue to improve precision cutting and using organ-protective buffer solutions to minimize procedure-associated cell death. PCLS prepared from virus-infected livers showed preserved functional metabolic properties. Importantly, in PCLS from adenovirus- and LCMV-infected livers we detected increased induction of apoptosis after TNF challenge ex vivo.
Conclusion: We conclude that PCLS can be used as model system to ex vivo characterize hepatocyte-intrinsic sensitivity to cell death. This may also enable researchers to characterize human hepatocyte sensitivity to apoptosis in PCLS prepared from patients with acute or chronic liver diseases.
Lay summary: Virus-infected hepatocytes in vivo show an increased sensitivity towards induction of cell death signaling through the TNF receptor. Studying this hepatocyte-intrinsic antiviral immune surveillance mechanism has been hampered by the absence of model systems that reciprocate the in vivo finding of increased apoptosis of virus-infected hepatocytes challenged with TNF. Herein, we report that an optimized protocol for generation of precision-cut liver slices can be used to study this hepatocyte-intrinsic surveillance mechanism ex vivo.
Keywords: IP3, inositol-3-phosphate; LCMV, lymphocytic choriomeningitis virus; PCLS, precision-cut liver slices; PLCg, phospholipase C gamma; ROS, reactive oxygen species; TNF, tumor necrosis factor; TNF-induced apoptosis; anti-viral immunity; precision-cut liver slices.
© 2022 The Authors.
Conflict of interest statement
The authors declare no conflict of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Protzer U., Maini M.K., Knolle P.A. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–213. - PubMed
-
- Maini M.K., Pallett L.J. Defective T-cell immunity in hepatitis B virus infection: why therapeutic vaccination needs a helping hand. Lancet Gastroenterol Hepatol. 2018;3:192–202. - PubMed
-
- Ficht X., Iannacone M. Immune surveillance of the liver by T cells. Sci Immunol. 2020;5 - PubMed
-
- Lampl S., Janas M.K., Donakonda S., Brugger M., Lohr K., Schneider A., et al. Reduced mitochondrial resilience enables non-canonical induction of apoptosis after TNF receptor signaling in virus-infected hepatocytes. J Hepatol. 2020;73(6):1347–1359. - PubMed
-
- Wohlleber D., Kashkar H., Gärtner K., Frings M.K., Odenthal M., Hegenbarth S., et al. TNF-induced target cell killing by CTL activated through cross-presentation. Cell Rep. 2012;2:478–487. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

