mPEG-PDLLA Micelles Potentiate Docetaxel for Intraperitoneal Chemotherapy in Ovarian Cancer Peritoneal Metastasis
- PMID: 35462938
- PMCID: PMC9019464
- DOI: 10.3389/fphar.2022.861938
mPEG-PDLLA Micelles Potentiate Docetaxel for Intraperitoneal Chemotherapy in Ovarian Cancer Peritoneal Metastasis
Abstract
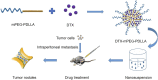
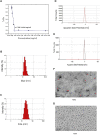
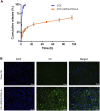
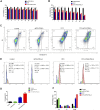
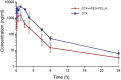
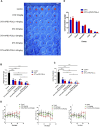
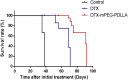
Ovarian cancer is the second most common cause of gynecological cancer death in women. It is usually diagnosed late and accompanied by peritoneal metastasis. For ovarian cancer with peritoneal metastasis, intraperitoneal (IP) chemotherapy can maintain a high drug concentration in the abdominal cavity and reduce local and systemic toxicity. Recently, docetaxel (DTX) has shown broad-spectrum antitumor activity against various malignant tumors, including ovarian cancer with peritoneal metastasis. However, DTX has limited clinical applications due to its poor water solubility, predisposition to hypersensitivity, fluid retention, and varying degrees of neurotoxicity. In this study, we prepared methoxy-poly(ethylene glycol)-block-poly(D,L-lactide) (mPEG-PDLLA) micelles loaded with DTX and developed an alternative, less toxic, more effective DTX formulation, without Tween 80, and evaluated its pharmacokinetics in the abdominal cavity and its efficacy in ovarian cancer with peritoneal metastasis. The mean diameter of DTX-mPEG-PDLLA was about 25 nm, and the pharmacokinetics of BALB/c mice via IP showed that the plasma exposure of DTX-mPEG-PDLLA was about four times lower than that of DTX. Importantly, DTX-mPEG-PDLLA was significantly more effective than DTX and prolonged the survival period in a SKOV-3 ovarian cancer peritoneal metastasis model. Moreover, the apoptosis rate was significantly increased in vitro. Based on these findings, it is expected that DTX-mPEG-PDLLA can enhance efficacy against ovarian cancer peritoneal metastasis, while reducing toxic side effects, and has the potential to be used in the clinical treatment of peritoneal metastatic cancer.
Keywords: DTX-mPEG-PDLLA micelles; intraperitoneal administration; ovarian cancer; peritoneal metastasis; pharmacokinetics.
Copyright © 2022 Zhang, Wang, Duan, Xu, Gao, Zhou, Xu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Chen J., Jiang Z., Zhang Y. S., Ding J., Chen X. (2021). Smart Transformable Nanoparticles for Enhanced Tumor Theranostics. Appl. Phys. Rev. 8, 041321. 10.1063/5.0061530 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

