Toward 3D-bioprinting of an endocrine pancreas: A building-block concept for bioartificial insulin-secreting tissue
- PMID: 35462988
- PMCID: PMC9024162
- DOI: 10.1177/20417314221091033
Toward 3D-bioprinting of an endocrine pancreas: A building-block concept for bioartificial insulin-secreting tissue
Abstract
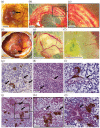
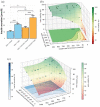
Three-dimensional bioprinting of an endocrine pancreas is a promising future curative treatment for patients with insulin secretion deficiency. In this study, we present an end-to-end concept from the molecular to the macroscopic level. Building-blocks for a hybrid scaffold device of hydrogel and functionalized polycaprolactone were manufactured by 3D-(bio)printing. Pseudoislet formation from INS-1 cells after bioprinting resulted in a viable and proliferative experimental model. Transcriptomics showed an upregulation of proliferative and ß-cell-specific signaling cascades, downregulation of apoptotic pathways, overexpression of extracellular matrix proteins, and VEGF induced by pseudoislet formation and 3D-culture. Co-culture with endothelial cells created a natural cellular niche with enhanced insulin secretion after glucose stimulation. Survival and function of pseudoislets after explantation and extensive scaffold vascularization of both hydrogel and heparinized polycaprolactone were demonstrated in vivo. Computer simulations of oxygen, glucose and insulin flows were used to evaluate scaffold architectures and Langerhans islets at a future perivascular transplantation site.
Keywords: Bioprinting; diabetes; endocrine pancreas; next-generation sequencing; tissue engineering.
© The Author(s) 2022.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.A.S., T.H. and H.G.K. are named inventors of a European patent application of University Hospital Heidelberg. Other authors declare that no competing financial interests exist.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

