Xenoimplant of Collagen Matrix Scaffold in Liver Tissue as a Niche for Liver Cells
- PMID: 35463025
- PMCID: PMC9022037
- DOI: 10.3389/fmed.2022.808191
Xenoimplant of Collagen Matrix Scaffold in Liver Tissue as a Niche for Liver Cells
Abstract
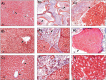
Hepatitis C virus-induced liver damage, chronic liver damage due to alcohol, and non-alcoholic liver disease-induced cellular alterations promote fibrosis, cirrhosis, and/or hepatocellular carcinoma. The recommended therapeutic option for advanced liver damage is liver transplantation. Extracellular matrix scaffolds have been evaluated as an alternative for tissue restoration. Studies on the biocompatibility and rejection of synthetic and natural scaffolds as an alternative to organ transplantation have been evaluated. Our group has recently described the xenoimplant of collagen matrix scaffold (CMS) in a rat model. However, no complete macroscopic and histological description of the liver parenchyma at the initial (day 3), intermediate (day 14), and advanced (day 21) stages has been obtained. In this study, we described and compared liver tissue from the CMS zone (CZ, CMS, and liver parenchyma), liver tissue from the normal zone (liver parenchyma close to the CMS), and basal tissue (resected tissue from the CMS implantation site). Our data strongly suggest that the collagen matrix xenoimplant is a good niche for hepatocytes, with no rejection, and does not affect liver function tests. The liver can regenerate after damage, but this capacity is inhibited in a chronic injury. At present, the use of CMS after liver damage has not been reported. This biomaterial could be a novel alternative in the field of regenerative medicine for liver diseases.
Keywords: animal model; cell niche; collagen matrix scaffold; liver; xenoimplant.
Copyright © 2022 Martinez-Castillo, León-Mancilla, Ramírez-Rico, Alfaro, Pérez-Torres, Díaz-Infante, García-Loya, Medina-Avila, Sanchez-Hernandez, Piña-Barba and Gutierrez-Reyes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

