Intervention Mechanism of Hunag-Lian Jie-Du Decoction on Canonical Wnt/ β-Catenin Signaling Pathway in Psoriasis Mouse Model
- PMID: 35463060
- PMCID: PMC9023143
- DOI: 10.1155/2022/3193572
Intervention Mechanism of Hunag-Lian Jie-Du Decoction on Canonical Wnt/ β-Catenin Signaling Pathway in Psoriasis Mouse Model
Abstract
Background: Psoriasis is a common chronic inflammatory skin disease with multifactor etiology, characterized by abnormal proliferation and differentiation of keratinocytes. Huang-Lian Jie-Du decoction (HLJDD) is a traditional Chinese medicine prescription with good clinical curative effect on psoriasis. However, its therapeutic mechanisms are still unclear.
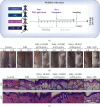
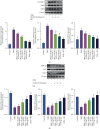
Methods: The psoriasis model of SKH-1 nude mice was established by imiquimod-induced and HLJDD gavage was given. Hematoxylin and eosin staining were used to evaluate pathological morphologies, and immunohistochemistry was used to detect the expressions of Wnt1, β-catenin, and c-Myc in psoriasis mice. Western blot was used to examine the expressions of Frizzled-2, LRP5/6, GSK-3β, APC, Axin2, TCF4, LEF1, cyclin D1, TBX3, EPHB2, and NOTUM enzyme.
Results: In this study, HLJDD reduced skin erythema and lesions, decreased the thickness of epidermal and downregulated the expressions of Wnt1, β-catenin, and c-Myc. Western blot results showed that HLJDD reduced the expressions of Wnt receptors Frizzled-2 and LRP5/6, and Wnt downstream target genes TCF4, LEF1, cyclin D1, TBX3, and EPHB2, while upregulated destruction complex proteins GSK-3β, APC, and Axin2.
Conclusions: HLJDD can effectively treat psoriasis and inhibit the Wnt/β-catenin signaling pathway at multiple stages.
Copyright © 2022 Xuesong Yang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Huang-Lian-Jie-Du Decoction Ameliorates Acute Ulcerative Colitis in Mice via Regulating NF-κB and Nrf2 Signaling Pathways and Enhancing Intestinal Barrier Function.Front Pharmacol. 2019 Nov 21;10:1354. doi: 10.3389/fphar.2019.01354. eCollection 2019. Front Pharmacol. 2019. PMID: 31849642 Free PMC article.
-
Mechanism of Huang-lian-Jie-du decoction and its effective fraction in alleviating acute ulcerative colitis in mice: Regulating arachidonic acid metabolism and glycerophospholipid metabolism.J Ethnopharmacol. 2020 Sep 15;259:112872. doi: 10.1016/j.jep.2020.112872. Epub 2020 May 14. J Ethnopharmacol. 2020. PMID: 32417423
-
Induced cortical neurogenesis after focal cerebral ischemia--Three active components from Huang-Lian-Jie-Du Decoction.J Ethnopharmacol. 2016 Feb 3;178:115-24. doi: 10.1016/j.jep.2015.12.001. Epub 2015 Dec 2. J Ethnopharmacol. 2016. PMID: 26657578
-
Huang-Lian Jie-Du decoction: a review on phytochemical, pharmacological and pharmacokinetic investigations.Chin Med. 2019 Dec 18;14:57. doi: 10.1186/s13020-019-0277-2. eCollection 2019. Chin Med. 2019. PMID: 31867052 Free PMC article. Review.
-
Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis.Front Immunol. 2018 Apr 13;9:745. doi: 10.3389/fimmu.2018.00745. eCollection 2018. Front Immunol. 2018. PMID: 29706964 Free PMC article. Review.
Cited by
-
The therapeutic effect of traditional Chinese medicine on breast cancer through modulation of the Wnt/β-catenin signaling pathway.Front Pharmacol. 2024 May 9;15:1401979. doi: 10.3389/fphar.2024.1401979. eCollection 2024. Front Pharmacol. 2024. PMID: 38783943 Free PMC article. Review.
-
Application of bioinformatics analysis and molecular docking to study the mechanism of Qingying decoction in treating psoriasis.Hereditas. 2025 Apr 7;162(1):52. doi: 10.1186/s41065-025-00421-8. Hereditas. 2025. PMID: 40197419 Free PMC article.
-
TOP2A Promotes Proliferation, Migration, and Inflammatory Response in M5-Treated Keratinocytes by Binding CTBP1 to Activate Wnt/β-Catenin Signaling.Cell Biochem Biophys. 2025 Jun;83(2):2101-2113. doi: 10.1007/s12013-024-01620-2. Epub 2024 Nov 20. Cell Biochem Biophys. 2025. PMID: 39565516
-
Cooling Blood and Detoxicating Formula Treats Psoriasis Through RHCG-Related Mechanisms.Int J Genomics. 2025 Jul 9;2025:5132158. doi: 10.1155/ijog/5132158. eCollection 2025. Int J Genomics. 2025. PMID: 40678620 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

