The Role of Inhibitory Interneurons in Circuit Assembly and Refinement Across Sensory Cortices
- PMID: 35463203
- PMCID: PMC9021723
- DOI: 10.3389/fncir.2022.866999
The Role of Inhibitory Interneurons in Circuit Assembly and Refinement Across Sensory Cortices
Abstract
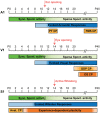
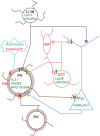
Sensory information is transduced into electrical signals in the periphery by specialized sensory organs, which relay this information to the thalamus and subsequently to cortical primary sensory areas. In the cortex, microcircuits constituted by interconnected pyramidal cells and inhibitory interneurons, distributed throughout the cortical column, form the basic processing units of sensory information underlying sensation. In the mouse, these circuits mature shortly after birth. In the first postnatal week cortical activity is characterized by highly synchronized spontaneous activity. While by the second postnatal week, spontaneous activity desynchronizes and sensory influx increases drastically upon eye opening, as well as with the onset of hearing and active whisking. This influx of sensory stimuli is fundamental for the maturation of functional properties and connectivity in neurons allocated to sensory cortices. In the subsequent developmental period, spanning the first five postnatal weeks, sensory circuits are malleable in response to sensory stimulation in the so-called critical periods. During these critical periods, which vary in timing and duration across sensory areas, perturbations in sensory experience can alter cortical connectivity, leading to long-lasting modifications in sensory processing. The recent advent of intersectional genetics, in vivo calcium imaging and single cell transcriptomics has aided the identification of circuit components in emergent networks. Multiple studies in recent years have sought a better understanding of how genetically-defined neuronal subtypes regulate circuit plasticity and maturation during development. In this review, we discuss the current literature focused on postnatal development and critical periods in the primary auditory (A1), visual (V1), and somatosensory (S1) cortices. We compare the developmental trajectory among the three sensory areas with a particular emphasis on interneuron function and the role of inhibitory circuits in cortical development and function.
Keywords: circuit; cortex; critical period; development; inhibition; interneuron; plasticity; sensory.
Copyright © 2022 Ferrer and De Marco García.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

