Lapatinib Suppresses HER2-Overexpressed Cholangiocarcinoma and Overcomes ABCB1- Mediated Gemcitabine Chemoresistance
- PMID: 35463361
- PMCID: PMC9033256
- DOI: 10.3389/fonc.2022.860339
Lapatinib Suppresses HER2-Overexpressed Cholangiocarcinoma and Overcomes ABCB1- Mediated Gemcitabine Chemoresistance
Abstract
Background: Recent breakthroughs in cholangiocarcinoma (CCA) genomics have led to the discovery of many unique identifying mutations, of which HER2 has been found to be overexpressed specifically in cases of extrahepatic CCA. However, whether or not lapatinib (an oral tyrosine kinase inhibitor selective for inhibition of HER2), or a combination of lapatinib and gemcitabine, exerts inhibitory effects on HER2-overexpressed CCA is still unclear.
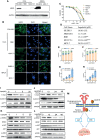
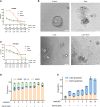
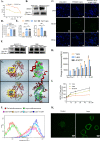
Methods: The effect of lapatinib and a lapatinib-gemcitabine combination treatment on CCA was determined using organoid and cell line models. Cell cycle arrest, apoptosis and proteins involving HER2-dependent downstream signaling pathways were analyzed to assess the effect of lapatinib on HER2+ CCA. The synergistic effect of lapatinib and gemcitabine was interpreted by docking analysis, ABCB1-associated ATPase assay, rhodamine transport assay and LC-MS/MS analyses.
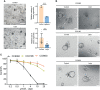
Results: dFdCTP, the active metabolite of gemcitabine, is proved to be the substrate of ABCB1 by docking analysis and ATPase assay. The upregulation of ABCB1 after gemcitabine treatment accounts for the resistance of gemcitabine. Lapatinib exerts a dual effect on HER2-overexpressed CCA, suppressing the growth of CCA cells by inhibiting HER2 and HER2-dependent downstream signaling pathways while inhibiting ABCB1 transporter function, allowing for the accumulation of active gemcitabine metabolites within cells.
Conclusions: Our data demonstrates that lapatinib can not only inhibit growth of CCA overexpressing HER2, but can also circumvent ABCB1-mediated chemoresistance after gemcitabine treatment. As such, this provides a preclinical rationale basis for further clinical investigation into the effectiveness of a combination treatment of lapatinib with gemcitabine in HER2-overexpressed CCA.
Keywords: ABCB1; HER2; chemoresistance; cholangiocarcinoma; gemcitabine; lapatinib.
Copyright © 2022 Bai, Guo, Liu, Chen, Lu, Zhang, Hong, Wang and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
HER2 as therapeutic target for overcoming ATP-binding cassette transporter-mediated chemoresistance in small cell lung cancer.Mol Cancer Ther. 2012 Apr;11(4):830-41. doi: 10.1158/1535-7163.MCT-11-0884. Epub 2012 Mar 2. Mol Cancer Ther. 2012. PMID: 22389470
-
A class I histone deacetylase inhibitor, entinostat, enhances lapatinib efficacy in HER2-overexpressing breast cancer cells through FOXO3-mediated Bim1 expression.Breast Cancer Res Treat. 2014 Jul;146(2):259-72. doi: 10.1007/s10549-014-3014-7. Epub 2014 Jun 12. Breast Cancer Res Treat. 2014. PMID: 24916181 Free PMC article.
-
MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells.Mol Cancer Ther. 2012 Mar;11(3):660-9. doi: 10.1158/1535-7163.MCT-11-0754. Epub 2012 Jan 11. Mol Cancer Ther. 2012. PMID: 22238368 Free PMC article.
-
Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases.Clin Ther. 2008 Aug;30(8):1426-47. doi: 10.1016/j.clinthera.2008.08.008. Clin Ther. 2008. PMID: 18803986 Review.
-
Lapatinib.Recent Results Cancer Res. 2018;211:19-44. doi: 10.1007/978-3-319-91442-8_2. Recent Results Cancer Res. 2018. PMID: 30069757 Review.
Cited by
-
Mechanisms underlying the changes in acetaldehyde dehydrogenase 1 in cholangiocarcinoma.J Cancer. 2023 Sep 25;14(17):3203-3213. doi: 10.7150/jca.86967. eCollection 2023. J Cancer. 2023. PMID: 37928420 Free PMC article. Review.
-
Recent progress in emerging molecular targeted therapies for intrahepatic cholangiocarcinoma.RSC Med Chem. 2025 Feb 6. doi: 10.1039/d4md00881b. Online ahead of print. RSC Med Chem. 2025. PMID: 39925737 Free PMC article. Review.
-
c-MET tyrosine kinase inhibitors reverse drug resistance mediated by the ATP-binding cassette transporter B1 (ABCB1) in cancer cells.3 Biotech. 2025 Jan;15(1):2. doi: 10.1007/s13205-024-04162-9. Epub 2024 Dec 4. 3 Biotech. 2025. PMID: 39650809
-
The Role of HER2 Status in the Biliary Tract Cancers.Cancers (Basel). 2023 May 5;15(9):2628. doi: 10.3390/cancers15092628. Cancers (Basel). 2023. PMID: 37174094 Free PMC article. Review.
-
Genomic mapping of copy number variations influencing immune response in breast cancer.Front Oncol. 2022 Sep 1;12:975437. doi: 10.3389/fonc.2022.975437. eCollection 2022. Front Oncol. 2022. PMID: 36119512 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

