Role of Nitric Oxide in Plant Senescence
- PMID: 35463429
- PMCID: PMC9022112
- DOI: 10.3389/fpls.2022.851631
Role of Nitric Oxide in Plant Senescence
Abstract
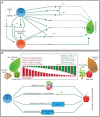
In plants senescence is the final stage of plant growth and development that ultimately leads to death. Plants experience age-related as well as stress-induced developmental ageing. Senescence involves significant changes at the transcriptional, post-translational and metabolomic levels. Furthermore, phytohormones also play a critical role in the programmed senescence of plants. Nitric oxide (NO) is a gaseous signalling molecule that regulates a plethora of physiological processes in plants. Its role in the control of ageing and senescence has just started to be elucidated. Here, we review the role of NO in the regulation of programmed cell death, seed ageing, fruit ripening and senescence. We also discuss the role of NO in the modulation of phytohormones during senescence and the significance of NO-ROS cross-talk during programmed cell death and senescence.
Keywords: ROS; ageing; nitric oxide; programmed cell death; senescence.
Copyright © 2022 Hussain, Shah, Ali and Yun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Andryka-Dudek P., Ciacka K., Wiśniewska A., Bogatek R., Gniazdowska A. (2019). Nitric oxide-induced dormancy removal of apple embryos is linked to alterations in expression of genes encoding ABA and JA biosynthetic or transduction pathways and RNA nitration. Int. J. Mol. Sci. 20:1007. doi: 10.3390/ijms20051007, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

