Health technology assessment of varicella vaccine in the Armed Forces
- PMID: 35463553
- PMCID: PMC9023555
- DOI: 10.1016/j.mjafi.2021.06.010
Health technology assessment of varicella vaccine in the Armed Forces
Abstract
Background: The Indian Armed Forces, on entry, vaccinates all cadets and recruits with varicella vaccine for the prevention of varicella. This health technology assessment (HTA) report puts forth evidence for HTA of varicella vaccination in the Armed Forces in various domains namely clinical, societal, ethical, economic, and legal.
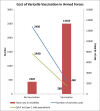
Methods: The policy question under each domain has been developed according to best-practice methods for HTA. The costs included were hospitalization cost due to varicella infection; training lost cost; the varicella vaccine cost; cost of the side effects of vaccine; and the outbreak investigation cost. The incremental cost-effectiveness ratio (ICER) for varicella cases averted and man-days saved, and quality-adjusted life years (QALYs) gained due to varicella vaccination strategy were calculated.
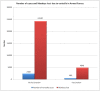
Results: Evidence suggests a reduction of 81% in hospitalization rates with 19392 man-days saved per 1 lakh population due to varicella vaccination strategy. The ICER for varicella cases averted is estimated to be Rs 56732/- per case averted and Rs 5687/- per man-day saved. QALYs gained due to two-dose varicella vaccination strategy is estimated to be 1152 per 1 lakh population with cost per QALY gained Rs 95735/-.
Conclusion: The study showed a large reduction in hospitalizations and consequently man-days lost after the introduction of the vaccination strategy. The QALYs was another aspect of importance brought out by this study. Thus, a two-dose vaccination strategy for varicella-zoster virus (VZV) for the Armed Forces trainees is a cost-effective policy.
Keywords: Chickenpox; Cost effectiveness; Health technology assessment; Varicella.
© 2022 Director General, Armed Forces Medical Services. Published by Elsevier, a division of RELX India Pvt. Ltd.
Conflict of interest statement
The authors have none to declare.
Figures
References
-
- Moffat J., Ku C.C., Zerboni L., et al. In: Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Arvin A., Campadelli-Fiume G., Mocarski E., et al., editors. Cambridge University Press; Cambridge: 2007. VZV: pathogenesis and the disease consequences of primary infection.https://www.ncbi.nlm.nih.gov/books/NBK47382/ Chapter 37. Available from: - PubMed
-
- Varicella (Chickenpox), Center for Disease Control and Prevention CDC, [accessed 14 May 2020] https://www.cdc.gov/chickenpox/about/transmission.html.
-
- Jane F Seward, Mona Marin, Varicella Disease Burden and Varicella Vaccines On behalf of the SAGE VZV Working Group, WHO SAGE Meeting April 2, 2014, CDC Immunization https://www.who.int/immunization/sage/meetings/2014/april/2_SAGE_April_V....
-
- Munech R., Nassim C., Nikku S. Sero-epidemiology of varicella. J Infect Dis. 1986;153:153–155. - PubMed
LinkOut - more resources
Full Text Sources