In Vitro Antiviral Activity of Potential Medicinal Plant Extracts Against Dengue and Chikungunya Viruses
- PMID: 35463636
- PMCID: PMC9021897
- DOI: 10.3389/fcimb.2022.866452
In Vitro Antiviral Activity of Potential Medicinal Plant Extracts Against Dengue and Chikungunya Viruses
Abstract
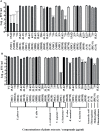
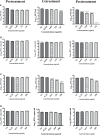
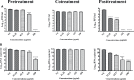
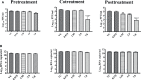
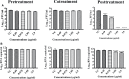
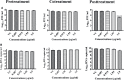
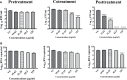
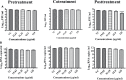
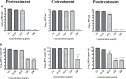
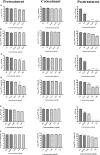
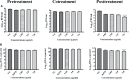
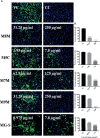
Dengue and chikungunya are two important mosquito-borne infections which are known to occur extensively in tropical and subtropical areas. Presently, there is no treatment for these viral diseases. In vitro antiviral screening of 25 extracts prepared from the plants of Vitex negundo, Plumeria alba, Ancistrocladus heyneanus, Bacopa monnieri, Anacardium occidentale, Cucurbita maxima, Simarouba glauca, and Embelia ribes using different solvents and four purified compounds (anacardic acid, chloroquinone, glaucarubinone, and methyl gallate) were carried out for their anti-dengue virus (DENV) and anti-chikungunya virus (CHIKV) activities. Maximum nontoxic concentrations of the chloroform, methanol, ethyl acetate, petroleum ether, dichloromethane, and hydroalcoholic extracts of eight plants were used. The antiviral activity was assessed by focus-forming unit assay, quantitative real-time RT-PCR, and immunofluorescence assays. Extracts from Plumeria alba, Ancistrocladus heyneanus, Bacopa monnieri, and Cucurbita maxima showed both anti-DENV and CHIKV activity while extract from Vitex negundo showed only anti-DENV activity. Among the purified compounds, anacardic acid, chloroquinone and methyl gallate showed anti-dengue activity while only methyl gallate had anti-chikungunya activity. The present study had identified the plant extracts with anti-dengue and anti-chikungunya activities, and these extracts can be further characterized for finding effective phytopharmaceutical drugs against dengue and chikungunya.
Keywords: antivirals; chikungunya virus; dengue virus; phytopharmaceuticals; plant extracts.
Copyright © 2022 Alagarasu, Patil, Kaushik, Chowdhury, Joshi, Hegde, Kakade, Hoti, Cherian and Parashar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Anggoro B., Istyastono E., Hariono M. (2020). Future Molecular Medicine From White Frangipani (Plumeria alba L.): A Review. J. Med. Plants Res. 14, 544–554. doi: 10.1016/j.phytochem.2012.07.023 - DOI
-
- Basri F., Sharma H. P., Firdaus S., Jain P., Ranjan A. (2014). A Review of Ethnomedicinal Plant-Vitex Negundo Linn. Int. J. Adv. Res. 2, 882–894.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

