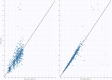
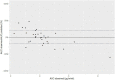
Can First-Dose Therapeutic Drug Monitoring Predict the Steady State Area Under the Blood Concentration-Time Curve of Busulfan in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation?
- PMID: 35463912
- PMCID: PMC9021690
- DOI: 10.3389/fped.2022.834773
Can First-Dose Therapeutic Drug Monitoring Predict the Steady State Area Under the Blood Concentration-Time Curve of Busulfan in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation?
Abstract
Busulfan has high intra-individual variability and possible time-dependent changes in clearance, which complicates therapeutic drug monitoring (TDM), as first dose sampling may not predict the steady state concentrations. In this study, we aimed to use Bayesian pharmacokinetic parameters estimated from the first dose to predict the steady state AUC for busulfan. This observational study was conducted among pediatric patients at King Abdullah Specialist Children's Hospital. From each patient, we collected six blood samples (2, 2.25, 2.5, 3, 4, and 6 h after the start of IV infusion of the first dose). A subset of patients were also sampled at the steady state. First, we modeled the data using only the first dose. The model was used to estimate the empirical Bayesian estimates of clearance for each individual patient, then we used the empirical Bayesian estimates of clearance to predict the AUC0-tau at steady state (i.e., predicted AUC0-tau). Steady state AUC0-tau was also calculated for patients sampled at steady state using the trapezoidal method using raw time concentration data; this was considered the reference AUC0-tau.. Then, we compared the AUC0-tau predicted using the Bayesian approach with the reference AUC0-tau values. We calculated bias and precision to assess predictability. In total we had 33 patients sampled after first dose and at steady state. Using the Bayesian approach to predict the AUC0-tau, bias was -2.8% and precision was 33%. This indicates that first dose concentrations cannot accurately predict steady state busulfan concentrations; therefore, follow-up TDM may be required for optimal dosing.
Keywords: Bayesian pharmacokinetics; TDM (therapeutic drug monitoring); area under the blood concentration-time curve (AUC); busulfan; pharmacokinetics.
Copyright © 2022 Alsultan, Albassam, Alturki, Alsultan, Essa, Almuzzaini and Alfadhel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Population pharmacokinetics of busulfan in Saudi pediatric patients undergoing hematopoietic stem cell transplantation.Int J Clin Pharm. 2020 Apr;42(2):703-712. doi: 10.1007/s11096-020-00989-3. Epub 2020 Mar 5. Int J Clin Pharm. 2020. PMID: 32140913
-
Intraindividual variability in busulfan pharmacokinetics in patients undergoing a bone marrow transplant: assessment of a test dose and first dose strategy.Anticancer Drugs. 2004 Jun;15(5):453-9. doi: 10.1097/01.cad.0000127145.50172.51. Anticancer Drugs. 2004. PMID: 15166618 Clinical Trial.
-
Iron Chelation With Deferasirox Increases Busulfan AUC During Conditioning Chemotherapy Prior to Allogeneic Stem Cell Transplantation.Transplant Cell Ther. 2022 Feb;28(2):115.e1-115.e5. doi: 10.1016/j.jtct.2021.11.003. Epub 2021 Nov 11. Transplant Cell Ther. 2022. PMID: 34775147
-
A limited sampling model for estimation of total and unbound mycophenolic acid (MPA) area under the curve (AUC) in hematopoietic cell transplantation (HCT).Ther Drug Monit. 2006 Jun;28(3):394-401. doi: 10.1097/01.ftd.0000211821.73231.8a. Ther Drug Monit. 2006. PMID: 16778725
-
Precision sirolimus dosing in children: The potential for model-informed dosing and novel drug monitoring.Front Pharmacol. 2023 Mar 20;14:1126981. doi: 10.3389/fphar.2023.1126981. eCollection 2023. Front Pharmacol. 2023. PMID: 37021042 Free PMC article. Review.
Cited by
-
Precision Oncology by Point-of-Care Therapeutic Drug Monitoring and Dosage Adjustment of Conventional Cytotoxic Chemotherapies: A Perspective.Pharmaceutics. 2023 Apr 19;15(4):1283. doi: 10.3390/pharmaceutics15041283. Pharmaceutics. 2023. PMID: 37111768 Free PMC article. Review.
References
-
- Palmer J, McCune JS, Perales MA, Marks D, Bubalo J, Mohty M, et al. Personalizing busulfan-based conditioning: considerations from the american society for blood and marrow transplantation practice guidelines committee. Biol Blood Marrow Transplant. (2016) 22:1915–25. 10.1016/j.bbmt.2016.07.013 - DOI - PubMed
-
- Hill BT, Rybicki LA, Urban TA, Lucena M, Jagadeesh D, Gerds AT, et al. Therapeutic dose monitoring of busulfan is associated with reduced risk of relapse in non-hodgkin lymphoma patients undergoing autologous stem cell transplantation. Biol Blood Marrow Transplant. (2020) 26:262–71. 10.1016/j.bbmt.2019.09.033 - DOI - PubMed
-
- Choong E, Uppugunduri CRS, Marino D, Kuntzinger M, Doffey-Lazeyras F, Lo Piccolo R, et al. Therapeutic drug monitoring of busulfan for the management of pediatric patients: cross-validation of methods and long-term performance. Ther Drug Monit. (2018) 40:84–92. 10.1097/ftd.0000000000000468 - DOI - PubMed
LinkOut - more resources
Full Text Sources