RANKL Impairs the TLR4 Pathway by Increasing TRAF6 and RANK Interaction in Macrophages
- PMID: 35463988
- PMCID: PMC9019442
- DOI: 10.1155/2022/7740079
RANKL Impairs the TLR4 Pathway by Increasing TRAF6 and RANK Interaction in Macrophages
Abstract
High serum levels of osteoprotegerin (OPG) are found in patients with obesity, type 2 diabetes, sepsis, or septic shock and are associated with a high mortality rate in stroke. The primary known function of OPG is to bind to the receptor activator of NF-κB ligand (RANKL), and by doing so, it inhibits the binding between RANKL and its receptor (RANK). TLR4 signaling in macrophages involves TRAF6 recruitment and contributes to low-grade chronic inflammation in adipose tissue. LPS is a classical activator of the TLR4 pathway and induces the expression of inflammatory cytokines in macrophages. We have previously observed that in the presence of RANKL, there is no LPS-induced activation of TLR4 in macrophages. In this study, we investigated the crosstalk between RANK and TLR4 pathways in macrophages stimulated with both RANKL and LPS to unveil the role of OPG in inflammatory processes. We found that RANKL inhibits TLR4 activation by binding to RANK, promoting the binding between TRAF6 and RANK, lowering TLR4 activation and the expression of proinflammatory mediators. Furthermore, high OPG levels aggravate inflammation by inhibiting RANKL. Our findings elect RANKL as a candidate for drug development as a way to mitigate the impact of obesity-induced inflammation in patients.
Copyright © 2022 Ryerson Fonseca Mota et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

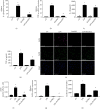
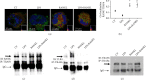
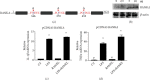

References
-
- Secchiero P., Corallini F., Pandolfi A., et al. An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction. The American Journal of Pathology . 2006;169(6):2236–2244. doi: 10.2353/ajpath.2006.060398. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

