Phenotype and Distribution of Immature Neurons in the Human Cerebral Cortex Layer II
- PMID: 35464133
- PMCID: PMC9027810
- DOI: 10.3389/fnana.2022.851432
Phenotype and Distribution of Immature Neurons in the Human Cerebral Cortex Layer II
Abstract
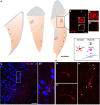
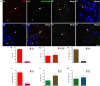
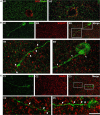
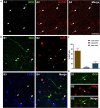
This work provides evidence of the presence of immature neurons in the human brain, specifically in the layer II of the cerebral cortex. Using surgical samples from epileptic patients and post-mortem tissue, we have found cells with different levels of dendritic complexity (type I and type II cells) expressing DCX and PSA-NCAM and lacking expression of the mature neuronal marker NeuN. These immature cells belonged to the excitatory lineage, as demonstrated both by the expression of CUX1, CTIP2, and TBR1 transcription factors and by the lack of the inhibitory marker GAD67. The type II cells had some puncta expressing inhibitory and excitatory synaptic markers apposed to their perisomatic and peridendritic regions and ultrastructural analysis suggest the presence of synaptic contacts. These cells did not present glial cell markers, although astroglial and microglial processes were found in close apposition to their somata and dendrites, particularly on type I cells. Our findings confirm the presence of immature neurons in several regions of the cerebral cortex of humans of different ages and define their lineage. The presence of some mature features in some of these cells suggests the possibility of a progressively integration as excitatory neurons, as described in the olfactory cortex of rodents.
Keywords: cerebral cortex; doublecortin; human brain; immature neurons; neurogenesis.
Copyright © 2022 Coviello, Gramuntell, Klimczak, Varea, Blasco-Ibañez, Crespo, Gutierrez and Nacher.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Olfactory bulbectomy, but not odor conditioned aversion, induces the differentiation of immature neurons in the adult rat piriform cortex.Neuroscience. 2011 May 5;181:18-27. doi: 10.1016/j.neuroscience.2011.03.004. Epub 2011 Mar 5. Neuroscience. 2011. PMID: 21382447
-
PSA-NCAM is Expressed in Immature, but not Recently Generated, Neurons in the Adult Cat Cerebral Cortex Layer II.Front Neurosci. 2011 Feb 15;5:17. doi: 10.3389/fnins.2011.00017. eCollection 2011. Front Neurosci. 2011. PMID: 21415912 Free PMC article.
-
Neurochemical Phenotype of Reelin Immunoreactive Cells in the Piriform Cortex Layer II.Front Cell Neurosci. 2016 Mar 10;10:65. doi: 10.3389/fncel.2016.00065. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27013976 Free PMC article.
-
Non-Newly Generated, "Immature" Neurons in the Sheep Brain Are Not Restricted to Cerebral Cortex.J Neurosci. 2018 Jan 24;38(4):826-842. doi: 10.1523/JNEUROSCI.1781-17.2017. Epub 2017 Dec 7. J Neurosci. 2018. PMID: 29217680 Free PMC article.
-
Migration of bone marrow progenitor cells in the adult brain of rats and rabbits.World J Stem Cells. 2016 Apr 26;8(4):136-57. doi: 10.4252/wjsc.v8.i4.136. World J Stem Cells. 2016. PMID: 27114746 Free PMC article. Review.
Cited by
-
Immature excitatory neurons in the amygdala come of age during puberty.Dev Cogn Neurosci. 2022 Aug;56:101133. doi: 10.1016/j.dcn.2022.101133. Epub 2022 Jul 10. Dev Cogn Neurosci. 2022. PMID: 35841648 Free PMC article.
-
Age-related changes in layer II immature neurons of the murine piriform cortex.Front Cell Neurosci. 2023 Jul 28;17:1205173. doi: 10.3389/fncel.2023.1205173. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37576566 Free PMC article.
-
Doublecortin-Expressing Neurons in Human Cerebral Cortex Layer II and Amygdala from Infancy to 100 Years Old.Mol Neurobiol. 2023 Jun;60(6):3464-3485. doi: 10.1007/s12035-023-03261-7. Epub 2023 Mar 6. Mol Neurobiol. 2023. PMID: 36879137
-
The effect of salidroside in promoting endogenous neural regeneration after cerebral ischemia/reperfusion involves notch signaling pathway and neurotrophic factors.BMC Complement Med Ther. 2024 Aug 1;24(1):293. doi: 10.1186/s12906-024-04597-w. BMC Complement Med Ther. 2024. PMID: 39090706 Free PMC article.
-
Adult neurogenesis and "immature" neurons in mammals: an evolutionary trade-off in plasticity?Brain Struct Funct. 2024 Nov;229(8):1775-1793. doi: 10.1007/s00429-023-02717-9. Epub 2023 Oct 13. Brain Struct Funct. 2024. PMID: 37833544 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

