Immunocytochemical Localization of Enzymes Involved in Dopamine, Serotonin, and Acetylcholine Synthesis in the Optic Neuropils and Neuroendocrine System of Eyestalks of Paralithodes camtschaticus
- PMID: 35464134
- PMCID: PMC9024244
- DOI: 10.3389/fnana.2022.844654
Immunocytochemical Localization of Enzymes Involved in Dopamine, Serotonin, and Acetylcholine Synthesis in the Optic Neuropils and Neuroendocrine System of Eyestalks of Paralithodes camtschaticus
Abstract
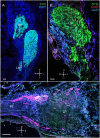
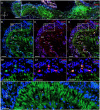
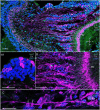
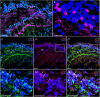
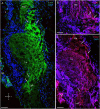
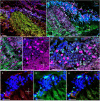
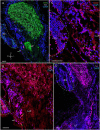
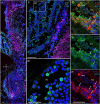
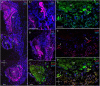
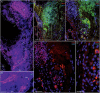
Identifying the neurotransmitters secreted by specific neurons in crustacean eyestalks is crucial to understanding their physiological roles. Here, we combined immunocytochemistry with confocal microscopy and identified the neurotransmitters dopamine (DA), serotonin (5-HT), and acetylcholine (ACh) in the optic neuropils and X-organ sinus gland (XO-SG) complex of the eyestalks of Paralithodes camtschaticus (red king crab). The distribution of Ach neurons was studied by choline acetyltransferase (ChAT) immunohistochemistry and compared with that of DA neurons examined in the same or adjacent sections by tyrosine hydroxylase (TH) immunohistochemistry. We detected 5-HT, TH, and ChAT in columnar, amacrine, and tangential neurons in the optic neuropils and established the presence of immunoreactive fibers and neurons in the terminal medulla in the XO region of the lateral protocerebrum. Additionally, we detected ChAT and 5-HT in the endogenous cells of the SG of P. camtschaticus for the first time. Furthermore, localization of 5-HT- and ChAT-positive cells in the SG indicated that these neurotransmitters locally modulate the secretion of neurohormones that are synthesized in the XO. These findings establish the presence of several neurotransmitters in the XO-SG complex of P. camtschaticus.
Keywords: acetylcholine; crustacea; dopamine; king crab; serotonin; sinus gland; tyrosine hydroxylase.
Copyright © 2022 Kotsyuba and Dyachuk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
[The distribution of catecholamine-containing neurons in the brain of Pagurus middendorffii and Paralithodes camtschaticus (Anomura, Decapoda)].Tsitologiia. 2012;54(6):515-21. Tsitologiia. 2012. PMID: 22997737 Russian.
-
First detection of Hematodinium sp. In spiny king crab Paralithodes brevipes, and new geographic areas for the parasite in tanner crab Chionoecetes bairdi, and red king crab Paralithodes camtschaticus.J Invertebr Pathol. 2021 Sep;184:107651. doi: 10.1016/j.jip.2021.107651. Epub 2021 Aug 1. J Invertebr Pathol. 2021. PMID: 34348127
-
Development of Serotonergic and Dopaminergic Neuronal Networks of the Central Nervous System in King Crab, Paralithodes camtschaticus.Biology (Basel). 2024 Jan 8;13(1):35. doi: 10.3390/biology13010035. Biology (Basel). 2024. PMID: 38248466 Free PMC article.
-
Localization of choline acetyltransferase-expressing neurons in Drosophila nervous system.Microsc Res Tech. 1999 Apr 15;45(2):65-79. doi: 10.1002/(SICI)1097-0029(19990415)45:2<65::AID-JEMT2>3.0.CO;2-0. Microsc Res Tech. 1999. PMID: 10332725 Review.
-
I. Serotonin (5-HT) within dopamine reward circuits signals open-field behavior. II. Basis for 5-HT--DA interaction in cocaine dysfunctional behavior.Neurosci Biobehav Rev. 1997 May;21(3):227-60. doi: 10.1016/s0149-7634(96)00048-6. Neurosci Biobehav Rev. 1997. PMID: 9168262 Review.
Cited by
-
3D Optical Reconstruction of the Nervous System of the Whole-Body Marine Invertebrates.Chem Biomed Imaging. 2023 Nov 10;1(9):852-863. doi: 10.1021/cbmi.3c00087. eCollection 2023 Dec 25. Chem Biomed Imaging. 2023. PMID: 39473836 Free PMC article.
References
-
- Andrew R. D., Saleuddin A. S. M. (1978). Structure and innervation of a crustacean neurosecretory cell. Can. J. Zool. 56 423–430. 10.1139/z78-060 - DOI
-
- Aréchiga H., Rodriguez-Sosa L. (2002). “Distributed circadian rhythmicity in the crustacean nervous system,” in The Crustacean Nervous System, ed. Wiese K. (Berlin: Springer; ), 113–122. 10.1007/978-3-662-04843-6_8 - DOI
LinkOut - more resources
Full Text Sources

