Chemistry and Bioactivity of Marine-Derived Bisabolane Sesquiterpenoids: A Review
- PMID: 35464222
- PMCID: PMC9021493
- DOI: 10.3389/fchem.2022.881767
Chemistry and Bioactivity of Marine-Derived Bisabolane Sesquiterpenoids: A Review
Abstract
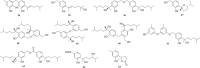
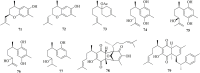
Natural products, characterized by intriguing scaffold diversity and structural complexity, as well as significant agricultural and medicinal activities, have been a valuable source of agrochemicals/drugs development and have historically made a huge contribution to pharmacotherapy. Structurally, bisabolanes are a family of naturally occurring sesquiterpenoids that featured a hexatomic ring core incorporating with eight continuous carbons, which cause high structural variability along the alkyl side chain to form abundant functionalities. Moreover, apart from their interesting structures, bisabolanes have shown multitudinous bioactivities. Bisabolanes are distributed in a variety of marine invertebrates, terrestrial plant, and microbial sources. Interestingly, bisabolanes characterized from marine environment possess unique characteristics both structurally and biologically. A total of 296 newly-discovered bisabolanes were searched. Among them, 94 members were isolated from marine organisms. This review particularly focuses on the new bisabolanes characterized from marine organisms (covering from 2000 to 2021), including marine-derived fungi, algae, soft corals, and sponges, with emphasis on the diversity of their chemical structures as well as the novelty and differences between terrestrial and marine sources. Moreover, a wide range of bioactivities of marine-derived bisabolanes, including antimicrobial, anti-inflammatory, enzyme inhibitory, and cytotoxic properties, are presented herein, which is considered to be a promising resource for the discovery of new drug leads and agrochemicals.
Keywords: biological activities; chemical diversity; lead compounds; marine-derived bisabolanes; sesquiterpenoids.
Copyright © 2022 Li, Liu, Yang, Li and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

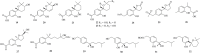

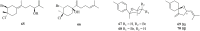



Similar articles
-
Research Advances of Bioactive Sesquiterpenoids Isolated from Marine-Derived Aspergillus sp.Molecules. 2022 Oct 30;27(21):7376. doi: 10.3390/molecules27217376. Molecules. 2022. PMID: 36364202 Free PMC article. Review.
-
Diterpenoids of Marine Organisms: Isolation, Structures, and Bioactivities.Mar Drugs. 2025 Mar 18;23(3):131. doi: 10.3390/md23030131. Mar Drugs. 2025. PMID: 40137317 Free PMC article. Review.
-
Sesquiterpenoids Specially Produced by Fungi: Structures, Biological Activities, Chemical and Biosynthesis (2015-2020).J Fungi (Basel). 2021 Nov 30;7(12):1026. doi: 10.3390/jof7121026. J Fungi (Basel). 2021. PMID: 34947008 Free PMC article. Review.
-
Chemistry and bioactivity of secondary metabolites from South China Sea marine fauna and flora: recent research advances and perspective.Acta Pharmacol Sin. 2022 Dec;43(12):3062-3079. doi: 10.1038/s41401-022-00980-w. Epub 2022 Sep 14. Acta Pharmacol Sin. 2022. PMID: 36104434 Free PMC article. Review.
-
Promising bioactive compounds from the marine environment and their potential effects on various diseases.J Genet Eng Biotechnol. 2022 Jan 26;20(1):14. doi: 10.1186/s43141-021-00290-4. J Genet Eng Biotechnol. 2022. PMID: 35080679 Free PMC article. Review.
Cited by
-
Nudibranchs as Sources of Marine Natural Products with Antitumor Activity: A Comprehensive Review.Mar Drugs. 2025 Aug 3;23(8):319. doi: 10.3390/md23080319. Mar Drugs. 2025. PMID: 40863636 Free PMC article. Review.
-
Chemistry and biology of marine-derived Trichoderma metabolites.Nat Prod Bioprospect. 2024 Feb 2;14(1):14. doi: 10.1007/s13659-024-00433-3. Nat Prod Bioprospect. 2024. PMID: 38302800 Free PMC article. Review.
-
Unlocking the Potential of Pyrrole: Recent Advances in New Pyrrole-Containing Compounds with Antibacterial Potential.Int J Mol Sci. 2024 Nov 29;25(23):12873. doi: 10.3390/ijms252312873. Int J Mol Sci. 2024. PMID: 39684580 Free PMC article. Review.
-
Marine Aspergillus: A Treasure Trove of Antimicrobial Compounds.Mar Drugs. 2023 Apr 28;21(5):277. doi: 10.3390/md21050277. Mar Drugs. 2023. PMID: 37233471 Free PMC article. Review.
-
Untargeted Metabolomics Approach for the Discovery of Salinity-Related Alkaloids in a Stony Coral-Derived Fungus Aspergillus terreus.Int J Mol Sci. 2024 Sep 30;25(19):10544. doi: 10.3390/ijms251910544. Int J Mol Sci. 2024. PMID: 39408873 Free PMC article.
References
-
- Cheng X., Li H., Wu P., Xu L., Xue J., Wei X. (2019). Two New Bisabolane-type Sesquiterpenoids from the Cooking Liquid of Curcuma Longa Rhizomes. Phytochemistry Lett. 29, 169–172. 10.1016/j.phytol.2018.12.004 - DOI
Publication types
LinkOut - more resources
Full Text Sources

