Roles of anti- and pro-oxidant potential of cinnamic acid and phenylpropanoid derivatives in modulating growth of cultured cells
- PMID: 35464248
- PMCID: PMC8994811
- DOI: 10.1007/s10068-022-01042-x
Roles of anti- and pro-oxidant potential of cinnamic acid and phenylpropanoid derivatives in modulating growth of cultured cells
Abstract
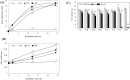
Cinnamic acid (CiA) and phenylpropanoid derivatives are widely distributed in plant foods. In this study, anti- and pro-oxidant properties of the derivatives and their roles in modulating cell growth were investigated. Ferulic acid, sinapinic acid, caffeic acid (CaA), and 3,4-dihydroxyhydrocinnamic acid (DHC) showed strong radical scavenging activities. They, except DHC, also performed considerable inhibitory effects on lipid peroxidation and reduced levels of intracellular reactive oxygen species (ROS). CaA and DHC, however, produced substantial amount of H2O2 with oxidative degradation in culture conditions. CaA and DHC (> 400 μM) showed potent cytotoxic effects which were abolished by superoxide dismutase/catalase; they significantly enhanced cell growth ROS-dependently at low levels (~ 100 μM). CiA derivatives without bearing hydroxyl group did not show any appreciable antioxidant activities. The results indicate that CiA derivatives with ortho-dihydroxyl group had strong anti- and pro-oxidant properties, which also play an important role in modulating cell growth.
Supplementary information: The online version contains supplementary material available at 10.1007/s10068-022-01042-x.
Keywords: Antioxidant; Cell growth; Cinnamic acid derivative; Phenylpropanoid; Prooxidant; Reactive oxygen species.
© The Korean Society of Food Science and Technology 2022.
Conflict of interest statement
Conflicts of interestThe authors have no conflict of interest to declare.
Figures


Similar articles
-
Enhanced lignin monomer production caused by cinnamic Acid and its hydroxylated derivatives inhibits soybean root growth.PLoS One. 2013 Dec 3;8(12):e80542. doi: 10.1371/journal.pone.0080542. eCollection 2013. PLoS One. 2013. PMID: 24312480 Free PMC article.
-
Structure-activity relationship of C6-C3 phenylpropanoids on xanthine oxidase-inhibiting and free radical-scavenging activities.Free Radic Biol Med. 2007 Dec 1;43(11):1541-51. doi: 10.1016/j.freeradbiomed.2007.08.018. Epub 2007 Sep 4. Free Radic Biol Med. 2007. PMID: 17964425
-
Modulation of intracellular reactive oxygen species level in chondrocytes by IGF-1, FGF, and TGF-beta1.Connect Tissue Res. 2007;48(3):149-58. doi: 10.1080/03008200701331516. Connect Tissue Res. 2007. PMID: 17522998
-
Melatonin: a well-documented antioxidant with conditional pro-oxidant actions.J Pineal Res. 2014 Sep;57(2):131-46. doi: 10.1111/jpi.12162. Epub 2014 Aug 6. J Pineal Res. 2014. PMID: 25060102 Review.
-
Antioxidant and antimicrobial activities of cinnamic acid derivatives.Mini Rev Med Chem. 2012 Jul;12(8):749-67. doi: 10.2174/138955712801264792. Mini Rev Med Chem. 2012. PMID: 22512578 Review.
Cited by
-
Dihydrocaffeic Acid-Is It the Less Known but Equally Valuable Phenolic Acid?Biomolecules. 2023 May 18;13(5):859. doi: 10.3390/biom13050859. Biomolecules. 2023. PMID: 37238728 Free PMC article. Review.
-
Enhancement of Solubility, Stability, Cellular Uptake, and Bioactivity of Curcumin by Polyvinyl Alcohol.Int J Mol Sci. 2024 Jun 6;25(11):6278. doi: 10.3390/ijms25116278. Int J Mol Sci. 2024. PMID: 38892468 Free PMC article.
-
Enhancing the Growth of Chili Plants and Soil Health: Synergistic Effects of Coconut Shell Biochar and Bacillus sp. Strain Ya-1 on Rhizosphere Microecology and Plant Metabolism.Int J Mol Sci. 2024 Oct 18;25(20):11231. doi: 10.3390/ijms252011231. Int J Mol Sci. 2024. PMID: 39457013 Free PMC article.
-
Modulation of Photosensitizing Responses in Cell Culture Environments by Different Medium Components.Int J Mol Sci. 2024 Sep 17;25(18):10016. doi: 10.3390/ijms251810016. Int J Mol Sci. 2024. PMID: 39337504 Free PMC article.
-
Molecular Mechanisms of Oxidative Stress Relief by CAPE in ARPE-19 Cells.Int J Mol Sci. 2023 Feb 10;24(4):3565. doi: 10.3390/ijms24043565. Int J Mol Sci. 2023. PMID: 36834980 Free PMC article.
References
-
- Anantharaju PG, Reddy DB, Padukudru MA, Chitturi CMK, Vimalambike MG, Madhunapantula SV. Induction of colon and cervical cancer cell death by cinnamic acid derivatives is mediated through the inhibition of histone deacetylases (HDAC) PloS One. 2017;12:0186208. doi: 10.1371/journal.pone.0186208. - DOI - PMC - PubMed
-
- Andjelković M, Van Camp J, De Meulenaer B, Depaemelaere G, Socaciu C, Verloo M, Verhe R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chemistry. 2006;98:23–31. doi: 10.1016/j.foodchem.2005.05.044. - DOI
LinkOut - more resources
Full Text Sources
