Loss of Function of the Neural Cell Adhesion Molecule NrCAM Regulates Differentiation, Proliferation and Neurogenesis in Early Postnatal Hypothalamic Tanycytes
- PMID: 35464310
- PMCID: PMC9022636
- DOI: 10.3389/fnins.2022.832961
Loss of Function of the Neural Cell Adhesion Molecule NrCAM Regulates Differentiation, Proliferation and Neurogenesis in Early Postnatal Hypothalamic Tanycytes
Abstract
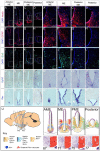
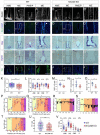
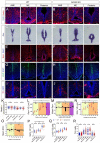
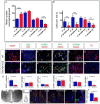
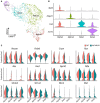
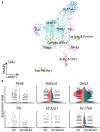
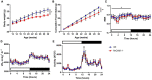
Hypothalamic tanycytes are neural stem and progenitor cells, but little is known of how they are regulated. Here we provide evidence that the cell adhesion molecule, NrCAM, regulates tanycytes in the adult niche. NrCAM is strongly expressed in adult mouse tanycytes. Immunohistochemical and in situ hybridization analysis revealed that NrCAM loss of function leads to both a reduced number of tanycytes and reduced expression of tanycyte-specific cell markers, along with a small reduction in tyrosine hydroxylase-positive arcuate neurons. Similar analyses of NrCAM mutants at E16 identify few changes in gene expression or cell composition, indicating that NrCAM regulates tanycytes, rather than early embryonic hypothalamic development. Neurosphere and organotypic assays support the idea that NrCAM governs cellular homeostasis. Single-cell RNA sequencing (scRNA-Seq) shows that tanycyte-specific genes, including a number that are implicated in thyroid hormone metabolism, show reduced expression in the mutant mouse. However, the mild tanycyte depletion and loss of markers observed in NrCAM-deficient mice were associated with only a subtle metabolic phenotype.
Keywords: NrCAM; astrocytes; hypothalamus; neural cell adhesion molecules; neurogenesis; radial glia; scRNA seq; tanycyte.
Copyright © 2022 Moore, Chinnaiya, Kim, Brown, Stewart, Robins, Dowsett, Muir, Travaglio, Lewis, Ebling, Blackshaw, Furley and Placzek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Developmental and functional relationships between hypothalamic tanycytes and embryonic radial glia.Front Neurosci. 2023 Jan 20;16:1129414. doi: 10.3389/fnins.2022.1129414. eCollection 2022. Front Neurosci. 2023. PMID: 36741057 Free PMC article. Review.
-
The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation.J Neurosci. 2014 Dec 10;34(50):16809-20. doi: 10.1523/JNEUROSCI.1711-14.2014. J Neurosci. 2014. PMID: 25505333 Free PMC article.
-
Tanycyte-Like Cells Derived From Mouse Embryonic Stem Culture Show Hypothalamic Neural Stem/Progenitor Cell Functions.Endocrinology. 2019 Jul 1;160(7):1701-1718. doi: 10.1210/en.2019-00105. Endocrinology. 2019. PMID: 31135891
-
Control of neurogenic competence in mammalian hypothalamic tanycytes.Sci Adv. 2021 May 28;7(22):eabg3777. doi: 10.1126/sciadv.abg3777. Print 2021 May. Sci Adv. 2021. PMID: 34049878 Free PMC article.
-
Irx3 and Irx5 - Novel Regulatory Factors of Postnatal Hypothalamic Neurogenesis.Front Neurosci. 2021 Nov 2;15:763856. doi: 10.3389/fnins.2021.763856. eCollection 2021. Front Neurosci. 2021. PMID: 34795556 Free PMC article. Review.
Cited by
-
Glucocorticoid receptor regulates protein chaperone, circadian clock and affective disorder genes in the zebrafish brain.Dis Model Mech. 2023 Sep 1;16(9):dmm050141. doi: 10.1242/dmm.050141. Epub 2023 Oct 2. Dis Model Mech. 2023. PMID: 37525888 Free PMC article.
-
Potential role of tanycyte-derived neurogenesis in Alzheimer's disease.Neural Regen Res. 2025 Jun 1;20(6):1599-1612. doi: 10.4103/NRR.NRR-D-23-01865. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934388 Free PMC article.
-
Reduced circulating NrCAM as a biomarker for fetal growth restriction.EBioMedicine. 2025 Aug;118:105854. doi: 10.1016/j.ebiom.2025.105854. Epub 2025 Jul 21. EBioMedicine. 2025. PMID: 40694862 Free PMC article.
-
Developmental and functional relationships between hypothalamic tanycytes and embryonic radial glia.Front Neurosci. 2023 Jan 20;16:1129414. doi: 10.3389/fnins.2022.1129414. eCollection 2022. Front Neurosci. 2023. PMID: 36741057 Free PMC article. Review.
-
Control of tuberal hypothalamic development and its implications in metabolic disorders.Nat Rev Endocrinol. 2025 Feb;21(2):118-130. doi: 10.1038/s41574-024-01036-1. Epub 2024 Sep 23. Nat Rev Endocrinol. 2025. PMID: 39313573 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

