Design and Evaluation of TIM-3-CD28 Checkpoint Fusion Proteins to Improve Anti-CD19 CAR T-Cell Function
- PMID: 35464394
- PMCID: PMC9018974
- DOI: 10.3389/fimmu.2022.845499
Design and Evaluation of TIM-3-CD28 Checkpoint Fusion Proteins to Improve Anti-CD19 CAR T-Cell Function
Abstract
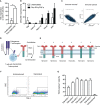
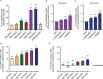
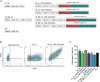
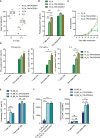
Therapeutic targeting of inhibitory checkpoint molecules in combination with chimeric antigen receptor (CAR) T cells is currently investigated in a variety of clinical studies for treatment of hematologic and solid malignancies. However, the impact of co-inhibitory axes and their therapeutic implication remains understudied for the majority of acute leukemias due to their low immunogenicity/mutational load. The inhibitory exhaustion molecule TIM-3 is an important marker for the interaction of T cells with leukemic cells. Moreover, inhibitory signals from malignant cells could be transformed into stimulatory signals by synthetic fusion molecules with extracellular inhibitory receptors fused to an intracellular stimulatory domain. Here, we designed a variety of different TIM-3-CD28 fusion proteins to turn inhibitory signals derived by TIM-3 engagement into T-cell activation through CD28. In the absence of anti-CD19 CAR, two TIM-3-CD28 fusion receptors with large parts of CD28 showed strongest responses in terms of cytokine secretion and proliferation upon stimulation with anti-CD3 antibodies compared to controls. We then combined these two novel TIM-3-CD28 fusion proteins with first- and second-generation anti-CD19 CAR T cells and found that the fusion receptor can increase proliferation, activation, and cytotoxic capacity of conventional anti-CD19 CAR T cells. These additionally armed CAR T cells showed excellent effector function. In terms of safety considerations, the fusion receptors showed exclusively increased cytokine release, when the CAR target CD19 was present. We conclude that combining checkpoint fusion proteins with anti-CD19 CARs has the potential to increase T-cell proliferation capacity with the intention to overcome inhibitory signals during the response against malignant cells.
Keywords: CAR T cells; CD19; CD28; TIM-3; acute lymphoblastic leukemia (ALL); checkpoint fusion proteins; pediatric leukemia.
Copyright © 2022 Blaeschke, Ortner, Stenger, Mahdawi, Apfelbeck, Habjan, Weißer, Kaeuferle, Willier, Kobold and Feuchtinger.
Conflict of interest statement
FB and SK: Patent applications have been filed in the field of immuno-oncology. SK has licensed IP to TCR2 Inc, Boston, USA and Carina Biotech, Adelaide, Australia. SK has received research support from TCR2 Inc, Boston, USA and Arcus Biosciences, San Francisco, USA. SK has received honoraria from TCR2 Inc, BMS, and Novartis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains.Mol Ther. 2017 Nov 1;25(11):2452-2465. doi: 10.1016/j.ymthe.2017.07.013. Epub 2017 Jul 27. Mol Ther. 2017. PMID: 28807568 Free PMC article.
-
Engineering of an Avidity-Optimized CD19-Specific Parallel Chimeric Antigen Receptor That Delivers Dual CD28 and 4-1BB Co-Stimulation.Front Immunol. 2022 Feb 9;13:836549. doi: 10.3389/fimmu.2022.836549. eCollection 2022. Front Immunol. 2022. PMID: 35222427 Free PMC article.
-
Treatment of patients with relapsed or refractory CD19+ lymphoid disease with T lymphocytes transduced by RV-SFG.CD19.CD28.4-1BBzeta retroviral vector: a unicentre phase I/II clinical trial protocol.BMJ Open. 2019 May 19;9(5):e026644. doi: 10.1136/bmjopen-2018-026644. BMJ Open. 2019. PMID: 31110096 Free PMC article. Clinical Trial.
-
Difference in Efficacy and Safety of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy Containing 4-1BB and CD28 Co-Stimulatory Domains for B-Cell Acute Lymphoblastic Leukemia.Cancers (Basel). 2023 May 15;15(10):2767. doi: 10.3390/cancers15102767. Cancers (Basel). 2023. PMID: 37345104 Free PMC article. Review.
-
Complexities in comparing the impact of costimulatory domains on approved CD19 CAR functionality.J Transl Med. 2023 Jul 30;21(1):515. doi: 10.1186/s12967-023-04372-4. J Transl Med. 2023. PMID: 37518011 Free PMC article. Review.
Cited by
-
A new story for an old challenge: Would flow cytometry beat molecular biology in monitoring chimeric antigen receptor T cell pharmacokinetics?Cytometry A. 2023 Jan;103(1):8-11. doi: 10.1002/cyto.a.24695. Epub 2022 Oct 18. Cytometry A. 2023. PMID: 36196578 Free PMC article. No abstract available.
-
Application and progress of CRISPR/Cas9 gene editing in B-cell lymphoma: a narrative review.Transl Cancer Res. 2024 Mar 31;13(3):1584-1595. doi: 10.21037/tcr-23-1146. Epub 2024 Mar 14. Transl Cancer Res. 2024. PMID: 38617522 Free PMC article. Review.
-
CAR-T-Cell Therapy Based on Immune Checkpoint Modulation in the Treatment of Hematologic Malignancies.Cell Transplant. 2024 Jan-Dec;33:9636897241293964. doi: 10.1177/09636897241293964. Cell Transplant. 2024. PMID: 39506457 Free PMC article. Review.
-
CAR-T cell therapy for hematological malignancies: Limitations and optimization strategies.Front Immunol. 2022 Sep 28;13:1019115. doi: 10.3389/fimmu.2022.1019115. eCollection 2022. Front Immunol. 2022. PMID: 36248810 Free PMC article. Review.
-
Immunotherapy for Pediatric Acute Lymphoblastic Leukemia: Recent Advances and Future Perspectives.Front Immunol. 2022 Jun 13;13:921894. doi: 10.3389/fimmu.2022.921894. eCollection 2022. Front Immunol. 2022. PMID: 35769486 Free PMC article. Review.
References
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. . T Cells Expressing CD19 Chimeric Antigen Receptors for Acute Lymphoblastic Leukaemia in Children and Young Adults: A Phase 1 Dose-Escalation Trial. Lancet (2015) 385:517–28. doi: 10.1016/S0140-6736(14)61403-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

