Thymic Microenvironment: Interactions Between Innate Immune Cells and Developing Thymocytes
- PMID: 35464404
- PMCID: PMC9024034
- DOI: 10.3389/fimmu.2022.885280
Thymic Microenvironment: Interactions Between Innate Immune Cells and Developing Thymocytes
Abstract
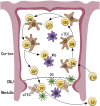
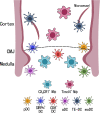
The thymus is a crucial organ for the development of T cells. T cell progenitors first migrate from the bone marrow into the thymus. During the journey to become a mature T cell, progenitors require interactions with many different cell types within the thymic microenvironment, such as stromal cells, which include epithelial, mesenchymal and other non-T-lineage immune cells. There are two crucial decision steps that are required for generating mature T cells: positive and negative selection. Each of these two processes needs to be performed efficiently to produce functional MHC-restricted T cells, while simultaneously restricting the production of auto-reactive T cells. In each step, there are various cell types that are required for the process to be carried out suitably, such as scavengers to clean up apoptotic thymocytes that fail positive or negative selection, and antigen presenting cells to display self-antigens during positive and negative selection. In this review, we will focus on thymic non-T-lineage immune cells, particularly dendritic cells and macrophages, and the role they play in positive and negative selection. We will also examine recent advances in the understanding of their participation in thymus homeostasis and T cell development. This review will provide a perspective on how the thymic microenvironment contributes to thymocyte differentiation and T cell maturation.
Keywords: T cell development; dendritic cell; macrophage; negative selection; positive selection; thymus; thymus repair.
Copyright © 2022 Wang and Zúñiga-Pflücker.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

