Genetic Engineering and Enrichment of Human NK Cells for CAR-Enhanced Immunotherapy of Hematological Malignancies
- PMID: 35464442
- PMCID: PMC9022481
- DOI: 10.3389/fimmu.2022.847008
Genetic Engineering and Enrichment of Human NK Cells for CAR-Enhanced Immunotherapy of Hematological Malignancies
Abstract
The great clinical success of chimeric antigen receptor (CAR) T cells has unlocked new levels of immunotherapy for hematological malignancies. Genetically modifying natural killer (NK) cells as alternative CAR immune effector cells is also highly promising, as NK cells can be transplanted across HLA barriers without causing graft-versus-host disease. Therefore, off-the-shelf usage of CAR NK cell products might allow to widely expand the clinical indications and to limit the costs of treatment per patient. However, in contrast to T cells, manufacturing suitable CAR NK cell products is challenging, as standard techniques for genetically engineering NK cells are still being defined. In this study, we have established optimal lentiviral transduction of primary human NK cells by systematically testing different internal promoters for lentiviral CAR vectors and comparing lentiviral pseudotypes and viral entry enhancers. We have additionally modified CAR constructs recognizing standard target antigens for acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) therapy-CD19, CD33, and CD123-to harbor a CD34-derived hinge region that allows efficient detection of transduced NK cells in vitro and in vivo and also facilitates CD34 microbead-assisted selection of CAR NK cell products to >95% purity for potential clinical usage. Importantly, as most leukemic blasts are a priori immunogenic for activated primary human NK cells, we developed an in vitro system that blocks the activating receptors NKG2D, DNAM-1, NKp30, NKp44, NKp46, and NKp80 on these cells and therefore allows systematic testing of the specific killing of CAR NK cells against ALL and AML cell lines and primary AML blasts. Finally, we evaluated in an ALL xenotransplantation model in NOD/SCID-gamma (NSG) mice whether human CD19 CAR NK cells directed against the CD19+ blasts are relying on soluble or membrane-bound IL15 production for NK cell persistence and also in vivo leukemia control. Hence, our study provides important insights into the generation of pure and highly active allogeneic CAR NK cells, thereby advancing adoptive cellular immunotherapy with CAR NK cells for human malignancies further.
Keywords: adoptive cellular immunotherapy; chimeric antigen receptor; genetic engineering; human NK cells; lentiviral vectors (LVS); transduction.
Copyright © 2022 Soldierer, Bister, Haist, Thivakaran, Cengiz, Sendker, Bartels, Thomitzek, Smorra, Hejazi, Uhrberg, Scheckenbach, Monzel, Wiek, Reinhardt, Niktoreh and Hanenberg.
Conflict of interest statement
HH and CW are inventors on a patent describing the CD34 hinge for CAR immune effector cells. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
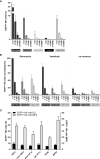
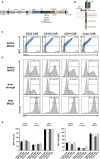


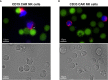


References
-
- Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, et al. . A Phase I Study of Nonmyeloablative Chemotherapy and Adoptive Transfer of Autologous Tumor Antigen-Specific T Lymphocytes in Patients With Metastatic Melanoma. J Immunother (2002) 25(3):243–51. doi: 10.1097/00002371-200205000-00007 - DOI - PMC - PubMed
-
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. . Eradication of B-Lineage Cells and Regression of Lymphoma in a Patient Treated With Autologous T Cells Genetically Engineered to Recognize CD19. Blood (2010) 116(20):4099–102. doi: 10.1182/blood-2010-04-281931 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

