Identification of the Transgene Integration Site and Host Genome Changes in MRP8-Cre/ires-EGFP Transgenic Mice by Targeted Locus Amplification
- PMID: 35464448
- PMCID: PMC9020256
- DOI: 10.3389/fimmu.2022.875991
Identification of the Transgene Integration Site and Host Genome Changes in MRP8-Cre/ires-EGFP Transgenic Mice by Targeted Locus Amplification
Abstract
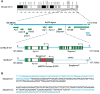
The MRP8-Cre-ires/EGFP transgenic mouse (Mrp8creTg, on C57BL/6J genetic background) is popular in immunological and hematological research for specifically expressing Cre recombinase and an EGFP reporter in neutrophils. It is often crossed with other transgenic lines carrying loxP-flanked genes to achieve restricted gene knockout in neutrophils. However, due to the way in which the line was created, basic knowledge about the MRP8-Cre-ires/EGFP transgene in the host genome, such as its integration site(s) and flanking sequences, remains largely unknown, hampering robust experimental design and data interpretation. Here we used a recently developed technique, targeted locus amplification (TLA) sequencing, to fill these knowledge gaps. We found that the MRP8-Cre-ires/EGFP transgene was integrated into chromosome 5 (5qG2) of the host mouse genome. This integration led to a 44 kb deletion of the host genomic sequence, resulting in complete deletion of Serpine1 and partial deletion of Ap1s1. Having determined the flanking sequences of the transgene, we designed a new genotyping protocol that can distinguish homozygous, heterozygous, and wildtype Mrp8creTg mice. To our surprise, crossing heterozygous mice produced no homozygous Mrp8creTg mice, most likely due to prenatal lethality resulting from disrupted Ap1s1 gene expression.
Keywords: MRP8-Cre transgenic mouse; Cre-loxP system; TLA sequencing; homozygous lethality; neutrophil.
Copyright © 2022 Wang, Zhang, Kambara, Dambrot, Xie, Zhao, Xu, Oneglia, Liu and Luo.
Conflict of interest statement
Authors CD and AO were employed by company Cergentis BV. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The need for Cre-loci controls in conditional mouse experiments: Mrp8-cre transgene predisposes mice to antibody-induced arthritis.Genes Immun. 2025 Apr;26(2):169-172. doi: 10.1038/s41435-024-00313-3. Epub 2024 Dec 4. Genes Immun. 2025. PMID: 39632992 Free PMC article.
-
Efficient mapping of transgene integration sites and local structural changes in Cre transgenic mice using targeted locus amplification.Nucleic Acids Res. 2017 May 5;45(8):e62. doi: 10.1093/nar/gkw1329. Nucleic Acids Res. 2017. PMID: 28053125 Free PMC article.
-
A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission.Biochem Biophys Res Commun. 1997 Aug 18;237(2):318-24. doi: 10.1006/bbrc.1997.7111. Biochem Biophys Res Commun. 1997. PMID: 9268708
-
Transgenic mouse with high Cre recombinase activity in all prostate lobes, seminal vesicle, and ductus deferens.Prostate. 2003 Oct 1;57(2):160-4. doi: 10.1002/pros.10283. Prostate. 2003. PMID: 12949940
-
An efficient system for tissue-specific overexpression of transgenes in podocytes in vivo.Am J Physiol Renal Physiol. 2005 Aug;289(2):F481-8. doi: 10.1152/ajprenal.00332.2004. Epub 2005 Mar 22. Am J Physiol Renal Physiol. 2005. PMID: 15784842
Cited by
-
LIFR regulates cholesterol-driven bidirectional hepatocyte-neutrophil cross-talk to promote liver regeneration.Nat Metab. 2024 Sep;6(9):1756-1774. doi: 10.1038/s42255-024-01110-y. Epub 2024 Aug 15. Nat Metab. 2024. PMID: 39147934 Free PMC article.
-
Neutrophil-specific Shp1 loss results in lethal pulmonary hemorrhage in mouse models of acute lung injury.J Clin Invest. 2024 Oct 1;134(24):e183161. doi: 10.1172/JCI183161. J Clin Invest. 2024. PMID: 39352872 Free PMC article.
-
The need for Cre-loci controls in conditional mouse experiments: Mrp8-cre transgene predisposes mice to antibody-induced arthritis.Genes Immun. 2025 Apr;26(2):169-172. doi: 10.1038/s41435-024-00313-3. Epub 2024 Dec 4. Genes Immun. 2025. PMID: 39632992 Free PMC article.
-
Neutrophil Granulopoiesis Optimized Through Ex Vivo Expansion of Hematopoietic Progenitors in Engineered 3D Gelatin Methacrylate Hydrogels.Adv Healthc Mater. 2024 Jun;13(14):e2301966. doi: 10.1002/adhm.202301966. Epub 2024 Feb 25. Adv Healthc Mater. 2024. PMID: 38345178 Free PMC article.
-
GM-CSF-mediated epithelial-immune cell cross-talk orchestrates pulmonary immunity to Aspergillus fumigatus.Sci Immunol. 2025 Mar 21;10(105):eadr0547. doi: 10.1126/sciimmunol.adr0547. Epub 2025 Mar 21. Sci Immunol. 2025. PMID: 40117345 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

