Randomized, Double-Blind, Reference-Controlled, Phase 2a Study Evaluating the Immunogenicity and Safety of OVX836, A Nucleoprotein-Based Influenza Vaccine
- PMID: 35464450
- PMCID: PMC9022189
- DOI: 10.3389/fimmu.2022.852904
Randomized, Double-Blind, Reference-Controlled, Phase 2a Study Evaluating the Immunogenicity and Safety of OVX836, A Nucleoprotein-Based Influenza Vaccine
Abstract
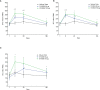
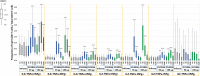
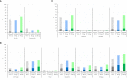
OVX836 is a recombinant protein-based vaccine targeting the highly conserved influenza nucleoprotein (NP), which aims to confer a broad-spectrum protection against influenza. In a Phase 1 study, OVX836, administered intramuscularly, has been found safe and immunogenic. The 90µg and 180µg dose levels were selected to be further evaluated in this randomized, monocenter, reference-controlled (Influvac Tetra™: quadrivalent seasonal influenza subunit vaccine), parallel group, double-blind, Phase 2a study in 300 healthy volunteers, aged 18-65 years, during the 2019/2020 flu season. Safety, influenza-like illness episodes (ILI; based on the Flu-PRO® questionnaire) and immunogenicity were assessed up to 180 days post-vaccination. OVX836 was safe and presented a reactogenicity profile similar to Influvac Tetra. It induced a significant increase in terms of NP-specific interferon-gamma (IFNγ) spot forming cells (SFCs), NP-specific CD4+ T-cells (essentially polyfunctional cells) and anti-NP IgG responses. OVX836 was superior to Influvac Tetra for all immunological parameters related to NP, and the 180µg dose was significantly superior to the 90µg dose for SFCs and CD4+ T-cells expressing IFNγ. Both the CD4+ T-cell and the anti-NP IgG responses persisted up to Day 180. An efficacy signal was observed with OVX836 at 180µg through reduction of ILI episodes occurring during the flu season as of 14 days post-vaccination. In conclusion, these results encourage further clinical evaluation of OVX836 in order to confirm the signal of efficacy on ILIs and/or laboratory-confirmed influenza cases. NCT04192500 (https://clinicaltrials.gov/ct2/show/study/NCT04192500).
Keywords: Influvac Tetra; OVX836; immunogenicity; influenza; nucleoprotein; safety; subunit; universal vaccine.
Copyright © 2022 Leroux-Roels, Waerlop, Tourneur, De Boever, Maes, Bruhwyler, Guyon-Gellin, Moris, Del Campo, Willems, Leroux-Roels, Le Vert and Nicolas.
Conflict of interest statement
JT, DG-G and JC are employees of Osivax. PW, PM, JB and GL-R are consultants who received fees from Osivax. AV and FN are shareholders and executive members of Osivax. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

