Th2-Oriented Immune Serum After SARS-CoV-2 Vaccination Does Not Enhance Infection In Vitro
- PMID: 35464483
- PMCID: PMC9024142
- DOI: 10.3389/fimmu.2022.882856
Th2-Oriented Immune Serum After SARS-CoV-2 Vaccination Does Not Enhance Infection In Vitro
Abstract
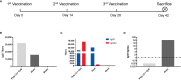
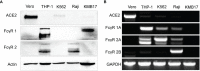
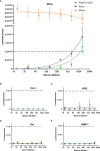
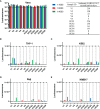
The relatively lower protection rate of the alum-adjuvanted inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines reminds us of the antibody-dependent enhancement (ADE) phenomenon observed in preclinical studies during the development of vaccines for Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1). In this study, using the S1 segment of the SARS-CoV-2 spike protein or inactivated whole SARS-CoV-2 virus as an antigen and aluminum as an adjuvant, the risk of ADE of infection with T helper 2 (Th2)-oriented immune serum from mice (N=6) and humans (N=5) was examined in immune cell lines, which show different expression patterns of Fc receptors. Neither the immune serum from alum-adjuvanted S1 subunit vaccines nor inactivated SARS-CoV-2 vaccination enhanced SARS-CoV-2 S pseudotyped virus infection in any of the tested cell lines in vitro. Because both of these Th2-oriented immune sera could block SARS-CoV-2 infection without ADE of infection, we speculate that the lower protection rate of the inactivated SARS-CoV-2 vaccine may be attributed to the lower neutralizing antibody titers induced or the pulmonary eosinophilic immunopathology accompanied by eosinophilic infiltration in the lungs upon virus exposure. Adjustment of the immunization schedule to elevate the neutralizing antibody levels and skew adjuvants toward Th1-oriented responses may be considered to increase the efficacies of both inactivated and spike protein-based subunit SARS-CoV-2 vaccines.
Trial registration: ClinicalTrials.gov NCT04510207 NCT04456595 NCT04582344 NCT04651790.
Keywords: Th2; aluminum adjuvant; antibody-dependent enhancement of infection; inactivated SARS-CoV-2 vaccine; spike protein subunit vaccine.
Copyright © 2022 Luan, Li, Wang, Cao, Yin, Lin and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology.J Virol. 2015 Mar;89(6):2995-3007. doi: 10.1128/JVI.02980-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520500 Free PMC article.
-
Intranasal vaccination induced cross-protective secretory IgA antibodies against SARS-CoV-2 variants with reducing the potential risk of lung eosinophilic immunopathology.Vaccine. 2022 Sep 29;40(41):5892-5903. doi: 10.1016/j.vaccine.2022.08.049. Epub 2022 Aug 26. Vaccine. 2022. PMID: 36064667 Free PMC article.
-
Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine.J Virol. 2014 Aug;88(15):8597-614. doi: 10.1128/JVI.00983-14. Epub 2014 May 21. J Virol. 2014. PMID: 24850731 Free PMC article.
-
[The Challenges of Vaccine Development Against Betacoronaviruses: Antibody Dependent Enhancement and Sendai Virus as a Possible Vaccine Vector].Mol Biol (Mosk). 2020 Nov-Dec;54(6):922-938. doi: 10.31857/S0026898420060154. Mol Biol (Mosk). 2020. PMID: 33276356 Review. Russian.
-
Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19.Hum Vaccin Immunother. 2020 Dec 1;16(12):3055-3060. doi: 10.1080/21645515.2020.1796425. Epub 2020 Aug 26. Hum Vaccin Immunother. 2020. PMID: 32845733 Free PMC article. Review.
Cited by
-
DNA origami vaccine (DoriVac) nanoparticles improve both humoral and cellular immune responses to infectious diseases.bioRxiv [Preprint]. 2025 Apr 4:2023.12.29.573647. doi: 10.1101/2023.12.29.573647. bioRxiv. 2025. PMID: 38260393 Free PMC article. Preprint.
-
Enhancement of SARS-CoV-2 N Antigen-Specific T Cell Functionality by Modulating the Autophagy-Mediated Signal Pathway in Mice.Viruses. 2023 Jun 2;15(6):1316. doi: 10.3390/v15061316. Viruses. 2023. PMID: 37376617 Free PMC article.
-
Antibody-dependent enhancement of coronaviruses.Int J Biol Sci. 2025 Feb 3;21(4):1686-1704. doi: 10.7150/ijbs.96112. eCollection 2025. Int J Biol Sci. 2025. PMID: 39990674 Free PMC article. Review.
-
Booster doses of an inactivated F genotype mumps vaccine enhance immunogenicity in mice.Vaccine X. 2024 Jan 13;17:100437. doi: 10.1016/j.jvacx.2024.100437. eCollection 2024 Mar. Vaccine X. 2024. PMID: 38317857 Free PMC article.
-
Protective roles and protective mechanisms of neutralizing antibodies against SARS-CoV-2 infection and their potential clinical implications.Front Immunol. 2023 Jan 19;14:1055457. doi: 10.3389/fimmu.2023.1055457. eCollection 2023. Front Immunol. 2023. PMID: 36742320 Free PMC article. Review.
References
-
- Agrawal AS, Tao X, Algaissi A, Garron T, Narayanan K, Peng B, et al. . Immunization With Inactivated Middle East Respiratory Syndrome Coronavirus Vaccine Leads to Lung Immunopathology on Challenge With Live Virus. Hum Vaccin Immunother (2016) 12(9):2351–6. doi: 10.1080/21645515.2016.1177688 - DOI - PMC - PubMed
-
- Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, et al. . A Double-Inactivated Severe Acute Respiratory Syndrome Coronavirus Vaccine Provides Incomplete Protection in Mice and Induces Increased Eosinophilic Proinflammatory Pulmonary Response Upon Challenge. J Virol (2011) 85(23):12201–15. doi: 10.1128/JVI.06048-11 - DOI - PMC - PubMed
-
- Jaume M, Yip MS, Cheung CY, Leung HL, Li PH, Kien F, et al. . Anti-Severe Acute Respiratory Syndrome Coronavirus Spike Antibodies Trigger Infection of Human Immune Cells via a pH- and Cysteine Protease-Independent FcgammaR Pathway. J Virol (2011) 85(20):10582–97. doi: 10.1128/JVI.00671-11 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

