Sialylation as an Important Regulator of Antibody Function
- PMID: 35464485
- PMCID: PMC9021442
- DOI: 10.3389/fimmu.2022.818736
Sialylation as an Important Regulator of Antibody Function
Abstract
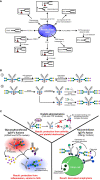
Antibodies play a critical role in linking the adaptive immune response to the innate immune system. In humans, antibodies are categorized into five classes, IgG, IgM, IgA, IgE, and IgD, based on constant region sequence, structure, and tropism. In serum, IgG is the most abundant antibody, comprising 75% of antibodies in circulation, followed by IgA at 15%, IgM at 10%, and IgD and IgE are the least abundant. All human antibody classes are post-translationally modified by sugars. The resulting glycans take on many divergent structures and can be attached in an N-linked or O-linked manner, and are distinct by antibody class, and by position on each antibody. Many of these glycan structures on antibodies are capped by sialic acid. It is well established that the composition of the N-linked glycans on IgG exert a profound influence on its effector functions. However, recent studies have described the influence of glycans, particularly sialic acid for other antibody classes. Here, we discuss the role of glycosylation, with a focus on terminal sialylation, in the biology and function across all antibody classes. Sialylation has been shown to influence not only IgG, but IgE, IgM, and IgA biology, making it an important and unappreciated regulator of antibody function.
Keywords: Immunoglobulins; antibody; glycosylation; in vitro glycoengineering; in vivo glyco engineering; sialic acid; sialylation.
Copyright © 2022 Vattepu, Sneed and Anthony.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

