The Role of the Oral Immune System in Oropharyngeal Candidiasis-Facilitated Invasion and Dissemination of Staphylococcus aureus
- PMID: 35464779
- PMCID: PMC9021398
- DOI: 10.3389/froh.2022.851786
The Role of the Oral Immune System in Oropharyngeal Candidiasis-Facilitated Invasion and Dissemination of Staphylococcus aureus
Abstract
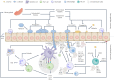
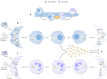
Candida albicans and Staphylococcus aureus account for most invasive fungal and bacterial bloodstream infections (BSIs), respectively. However, the initial point of invasion responsible for S. aureus BSIs is often unclear. Recently, C. albicans has been proposed to mediate S. aureus invasion of immunocompromised hosts during co-colonization of oral mucosal surfaces. The status of the oral immune system crucially contributes to this process in two distinct ways: firstly, by allowing invasive C. albicans growth during dysfunction of extra-epithelial immunity, and secondly following invasion by some remaining function of intra-epithelial immunity. Immunocompromised individuals at risk of developing invasive oral C. albicans infections could, therefore, also be at risk of contracting concordant S. aureus BSIs. Considering the crucial contribution of both oral immune function and dysfunction, the aim of this review is to provide an overview of relevant aspects of intra and extra-epithelial oral immunity and discuss predominant immune deficiencies expected to facilitate C. albicans induced S. aureus BSIs.
Keywords: Candida albicans; Staphylococcus aureus; bloodstream infection (BSI); immunocompromised; oral.
Copyright © 2022 Pasman, Krom, Zaat and Brul.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflictof interest.
Figures
Similar articles
-
Clinical characteristics, risk factors and outcomes of mixed Candida albicans/bacterial bloodstream infections.BMC Infect Dis. 2020 Nov 6;20(1):810. doi: 10.1186/s12879-020-05536-z. BMC Infect Dis. 2020. PMID: 33158426 Free PMC article.
-
Enhanced Virulence of Candida albicans by Staphylococcus aureus: Evidence in Clinical Bloodstream Infections and Infected Zebrafish Embryos.J Fungi (Basel). 2021 Dec 20;7(12):1099. doi: 10.3390/jof7121099. J Fungi (Basel). 2021. PMID: 34947081 Free PMC article.
-
Adhesion of Staphylococcus aureus to Candida albicans During Co-Infection Promotes Bacterial Dissemination Through the Host Immune Response.Front Cell Infect Microbiol. 2021 Feb 2;10:624839. doi: 10.3389/fcimb.2020.624839. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33604309 Free PMC article.
-
Innate Immunity and Saliva in Candida albicans-mediated Oral Diseases.J Dent Res. 2016 Apr;95(4):365-71. doi: 10.1177/0022034515625222. Epub 2016 Jan 8. J Dent Res. 2016. PMID: 26747422 Free PMC article. Review.
-
Immunity to Candida.Oral Dis. 2002;8 Suppl 2:69-75. doi: 10.1034/j.1601-0825.2002.00015.x. Oral Dis. 2002. PMID: 12164664 Review.
Cited by
-
Altered oral microbiome in Sudanese Toombak smokeless tobacco users carries a newly emerging risk of squamous cell carcinoma development and progression.Sci Rep. 2023 Apr 24;13(1):6645. doi: 10.1038/s41598-023-32892-y. Sci Rep. 2023. PMID: 37095112 Free PMC article.
-
Epidemiology risk factors and antifungal resistance patterns of Candida in cancer patients in Jiangxi China.Front Microbiol. 2025 Jul 22;16:1630226. doi: 10.3389/fmicb.2025.1630226. eCollection 2025. Front Microbiol. 2025. PMID: 40766085 Free PMC article.
-
A customizable and defined medium supporting culturing of Candida albicans, Staphylococcus aureus, and human oral epithelial cells.Appl Environ Microbiol. 2024 Aug 21;90(8):e0036024. doi: 10.1128/aem.00360-24. Epub 2024 Jul 29. Appl Environ Microbiol. 2024. PMID: 39072650 Free PMC article.
-
Microbial functional pathways based on metatranscriptomic profiling enable effective saliva-based health assessments for precision wellness.Comput Struct Biotechnol J. 2024 Jan 29;23:834-842. doi: 10.1016/j.csbj.2024.01.018. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 38328005 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

