The Type VI Secretion Systems in Plant-Beneficial Bacteria Modulate Prokaryotic and Eukaryotic Interactions in the Rhizosphere
- PMID: 35464916
- PMCID: PMC9022076
- DOI: 10.3389/fmicb.2022.843092
The Type VI Secretion Systems in Plant-Beneficial Bacteria Modulate Prokaryotic and Eukaryotic Interactions in the Rhizosphere
Abstract
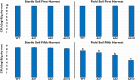
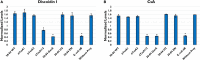
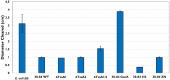
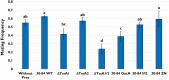
Rhizosphere colonizing plant growth promoting bacteria (PGPB) increase their competitiveness by producing diffusible toxic secondary metabolites, which inhibit competitors and deter predators. Many PGPB also have one or more Type VI Secretion System (T6SS), for the delivery of weapons directly into prokaryotic and eukaryotic cells. Studied predominantly in human and plant pathogens as a virulence mechanism for the delivery of effector proteins, the function of T6SS for PGPB in the rhizosphere niche is poorly understood. We utilized a collection of Pseudomonas chlororaphis 30-84 mutants deficient in one or both of its two T6SS and/or secondary metabolite production to examine the relative importance of each T6SS in rhizosphere competence, bacterial competition, and protection from bacterivores. A mutant deficient in both T6SS was less persistent than wild type in the rhizosphere. Both T6SS contributed to competitiveness against other PGPB or plant pathogenic strains not affected by secondary metabolite production, but only T6SS-2 was effective against strains lacking their own T6SS. Having at least one T6SS was also essential for protection from predation by several eukaryotic bacterivores. In contrast to diffusible weapons that may not be produced at low cell density, T6SS afford rhizobacteria an additional, more immediate line of defense against competitors and predators.
Keywords: GacS/GacA; PGPB (plant growth-promoting bacteria); Pseudomonas; T6SS; bacterivores; competition; rhizosphere ecology.
Copyright © 2022 Boak, Kirolos, Pan, Pierson and Pierson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

