Excitatory and Inhibitory Synaptic Imbalance Caused by Brain-Derived Neurotrophic Factor Deficits During Development in a Valproic Acid Mouse Model of Autism
- PMID: 35465089
- PMCID: PMC9019547
- DOI: 10.3389/fnmol.2022.860275
Excitatory and Inhibitory Synaptic Imbalance Caused by Brain-Derived Neurotrophic Factor Deficits During Development in a Valproic Acid Mouse Model of Autism
Abstract
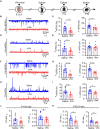
Environmental factors, such as medication during pregnancy, are one of the major causes of autism spectrum disorder (ASD). Valproic acid (VPA) intake during pregnancy has been reported to dramatically elevate autism risk in offspring. Recently, researchers have proposed that VPA exposure could induce excitatory or inhibitory synaptic dysfunction. However, it remains to be determined whether and how alterations in the excitatory/inhibitory (E/I) balance contribute to VPA-induced ASD in a mouse model. In the present study, we explored changes in the E/I balance during different developmental periods in a VPA mouse model. We found that typical markers of pre- and postsynaptic excitatory and inhibitory function involved in E/I balance markedly decreased during development, reflecting difficulties in the development of synaptic plasticity in VPA-exposed mice. The expression of brain-derived neurotrophic factor (BDNF), a neurotrophin that promotes the formation and maturation of glutamatergic and GABAergic synapses during postnatal development, was severely reduced in the VPA-exposed group. Treatment with exogenous BDNF during the critical E/I imbalance period rescued synaptic functions and autism-like behaviors, such as social defects. With these results, we experimentally showed that social dysfunction in the VPA mouse model of autism might be caused by E/I imbalance stemming from BDNF deficits during the developmental stage.
Keywords: BDNF; E/I balance; autism; excitatory synapse; inhibitory synapse; synaptic development; valproic acid.
Copyright © 2022 Qi, Chen, Mao, Hu, Ge, Ma, Ren, Xue, Wang and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Aguado F., Carmona M. A., Pozas E., Aguilo A., Martinez-Guijarro F. J., Alcantara S., et al. (2003). BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl- co-transporter KCC2. Development 130 1267–1280. 10.1242/dev.00351 - DOI - PubMed
LinkOut - more resources
Full Text Sources

