Beyond the Channels: Adhesion Functions of Aquaporin 0 and Connexin 50 in Lens Development
- PMID: 35465319
- PMCID: PMC9022433
- DOI: 10.3389/fcell.2022.866980
Beyond the Channels: Adhesion Functions of Aquaporin 0 and Connexin 50 in Lens Development
Abstract
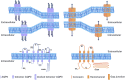
Lens, an avascular tissue involved in light transmission, generates an internal microcirculatory system to promote ion and fluid circulation, thus providing nutrients to internal lens cells and excreting the waste. This unique system makes up for the lack of vasculature and distinctively maintains lens homeostasis and lens fiber cell survival through channels of connexins and other transporters. Aquaporins (AQP) and connexins (Cx) comprise the majority of channels in the lens microcirculation system and are, thus, essential for lens development and transparency. Mutations of AQPs and Cxs result in abnormal channel function and cataract formation. Interestingly, in the last decade or so, increasing evidence has emerged suggesting that in addition to their well-established channel functions, AQP0 and Cx50 play pivotal roles through channel-independent actions in lens development and transparency. Specifically, AQP0 and Cx50 have been shown to have a unique cell adhesion function that mediates lens development and transparency. Precise regulation of cell-matrix and cell-cell adhesion is necessary for cell migration, a critical process during lens development. This review will provide recent advances in basic research of cell adhesion mediated by AQP0 and Cx50.
Keywords: aquaporin; cell adhesion; connexin; lens cell differentiation; lens development; lens transparency.
Copyright © 2022 Li, Quan, Gu and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Connexin 50 and AQP0 are Essential in Maintaining Organization and Integrity of Lens Fibers.Invest Ophthalmol Vis Sci. 2019 Sep 3;60(12):4021-4032. doi: 10.1167/iovs.18-26270. Invest Ophthalmol Vis Sci. 2019. PMID: 31560767 Free PMC article.
-
Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50.J Cell Sci. 2011 Jan 15;124(Pt 2):198-206. doi: 10.1242/jcs.072652. Epub 2010 Dec 20. J Cell Sci. 2011. PMID: 21172802 Free PMC article.
-
Connexin 50 Functions as an Adhesive Molecule and Promotes Lens Cell Differentiation.Sci Rep. 2017 Jul 13;7(1):5298. doi: 10.1038/s41598-017-05647-9. Sci Rep. 2017. PMID: 28706245 Free PMC article.
-
Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts.Handb Exp Pharmacol. 2009;(190):265-97. doi: 10.1007/978-3-540-79885-9_14. Handb Exp Pharmacol. 2009. PMID: 19096783 Review.
-
Focus on lens connexins.BMC Cell Biol. 2017 Jan 17;18(Suppl 1):6. doi: 10.1186/s12860-016-0116-6. BMC Cell Biol. 2017. PMID: 28124626 Free PMC article. Review.
Cited by
-
Abnormal function of EPHA2/p.R957P mutant in congenital cataract.Int J Ophthalmol. 2024 Jun 18;17(6):1007-1017. doi: 10.18240/ijo.2024.06.04. eCollection 2024. Int J Ophthalmol. 2024. PMID: 38895685 Free PMC article.
-
Charged multivesicular body protein 4b forms complexes with gap junction proteins during lens fiber cell differentiation.FASEB J. 2023 Apr;37(4):e22801. doi: 10.1096/fj.202201368RR. FASEB J. 2023. PMID: 36880430 Free PMC article.
-
Novel MIP gene mutation causes autosomal-dominant congenital cataract.Int J Ophthalmol. 2024 Mar 18;17(3):454-465. doi: 10.18240/ijo.2024.03.06. eCollection 2024. Int J Ophthalmol. 2024. PMID: 38721506 Free PMC article.
-
Ankyrin-B is required for the establishment and maintenance of lens cytoarchitecture, mechanics and clarity.J Cell Sci. 2024 Dec 15;137(24):jcs262349. doi: 10.1242/jcs.262349. Epub 2024 Dec 18. J Cell Sci. 2024. PMID: 39558792
-
Structural basis of aquaporin-4 autoantibody binding in neuromyelitis optica.Sci Adv. 2025 Feb 21;11(8):eadq7560. doi: 10.1126/sciadv.adq7560. Epub 2025 Feb 21. Sci Adv. 2025. PMID: 39982991 Free PMC article.
References
-
- Berry V., Ionides A., Pontikos N., Moghul I., Moore A. T., Quinlan R. A., et al. (2020). Whole Exome Sequencing Reveals Novel and Recurrent Disease-Causing Variants in Lens Specific Gap Junctional Protein Encoding Genes Causing Congenital Cataract. Genes (Basel) 11. 10.3390/genes11050512 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

