A Putative Plasmodium RNA-Binding Protein Plays a Critical Role in Female Gamete Fertility and Parasite Transmission to the Mosquito Vector
- PMID: 35465336
- PMCID: PMC9022223
- DOI: 10.3389/fcell.2022.825247
A Putative Plasmodium RNA-Binding Protein Plays a Critical Role in Female Gamete Fertility and Parasite Transmission to the Mosquito Vector
Abstract
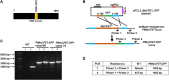
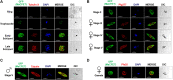
Plasmodium falciparum sexual stage gametocytes are critical for parasite transmission from the human host to the mosquito vector. Mature gametocytes generate fertile male (micro-) or female (macro-) gametes upon activation inside the mosquito midgut. While a number of parasite genes have been described that are critical for P. falciparum gametogenesis and fertility, no parasite gene has been shown to have a unique function in macrogametes. The genome of P. falciparum encodes numerous RNA-binding proteins. We identified a novel protein containing a putative RNA-binding domain, which we named Macrogamete-Contributed Factor Essential for Transmission (MaCFET). This protein is expressed in the asexual and sexual stages. Parasites that carry a deletion of MaCFET (Pfmacfet¯), developed normally as asexual stages, indicating that its function is not essential for the asexual proliferation of the parasite in vitro. Furthermore, Pfmacfet¯ male and female gametocytes developed normally and underwent activation to form microgametes and macrogametes. However, by utilizing genetic crosses, we demonstrate that Pfmacfet¯ parasites suffer a complete female-specific defect in successful fertilization. Therefore, PfMaCFET is a critical female-contributed factor for parasite transmission to the mosquito. Based on its putative RNA-binding properties, PfMaCFET might be in involved in the regulation of mRNAs that encode female-specific functions for fertilization or female-contributed factors needed post fertilization.
Keywords: fertility; gamete; gametocyte; mosquito; transmission.
Copyright © 2022 Kumar, Abatiyow, Haile, Oualim, Leeb, Vaughan and Kappe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

