Systematic characterization of wing mechanosensors that monitor airflow and wing deformations
- PMID: 35465360
- PMCID: PMC9018384
- DOI: 10.1016/j.isci.2022.104150
Systematic characterization of wing mechanosensors that monitor airflow and wing deformations
Abstract
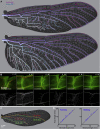
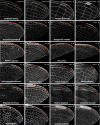
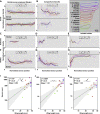
Animal wings deform during flight in ways that can enhance lift, facilitate flight control, and mitigate damage. Monitoring the structural and aerodynamic state of the wing is challenging because deformations are passive, and the flow fields are unsteady; it requires distributed mechanosensors that respond to local airflow and strain on the wing. Without a complete map of the sensor arrays, it is impossible to model control strategies underpinned by them. Here, we present the first systematic characterization of mechanosensors on the dragonfly's wings: morphology, distribution, and wiring. By combining a cross-species survey of sensor distribution with quantitative neuroanatomy and a high-fidelity finite element analysis, we show that the mechanosensors are well placed to perceive features of the wing dynamics relevant to flight. This work describes the wing sensory apparatus in its entirety and advances our understanding of the sensorimotor loop that facilitates exquisite flight control in animals with highly deformable wings.
Keywords: Animal physiology; Biomechanics; Entomology; Sensory neuroscience.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Spatial distribution of campaniform sensilla mechanosensors on wings: form, function, and phylogeny.Curr Opin Insect Sci. 2021 Dec;48:8-17. doi: 10.1016/j.cois.2021.06.002. Epub 2021 Jun 24. Curr Opin Insect Sci. 2021. PMID: 34175464 Review.
-
Ultrastructure of dragonfly wing veins: composite structure of fibrous material supplemented by resilin.J Anat. 2015 Oct;227(4):561-82. doi: 10.1111/joa.12362. J Anat. 2015. PMID: 26352411 Free PMC article.
-
Structural dynamics and aerodynamics measurements of biologically inspired flexible flapping wings.Bioinspir Biomim. 2011 Mar;6(1):016009. doi: 10.1088/1748-3182/6/1/016009. Epub 2011 Feb 22. Bioinspir Biomim. 2011. PMID: 21339627
-
Gyroscopic sensing in the wings of the hawkmoth Manduca sexta: the role of sensor location and directional sensitivity.Bioinspir Biomim. 2015 Oct 6;10(5):056013. doi: 10.1088/1748-3190/10/5/056013. Bioinspir Biomim. 2015. PMID: 26440705
-
Unsteady aerodynamics of insect flight.Symp Soc Exp Biol. 1995;49:109-29. Symp Soc Exp Biol. 1995. PMID: 8571220 Review.
Cited by
-
Nonuniform structural properties of wings confer sensing advantages.J R Soc Interface. 2023 Mar;20(200):20220765. doi: 10.1098/rsif.2022.0765. Epub 2023 Mar 22. J R Soc Interface. 2023. PMID: 36946090 Free PMC article.
-
Lessons from natural flight for aviation: then, now and tomorrow.J Exp Biol. 2023 Apr 25;226(Suppl_1):jeb245409. doi: 10.1242/jeb.245409. Epub 2023 Apr 17. J Exp Biol. 2023. PMID: 37066792 Free PMC article. Review.
-
Embodied airflow sensing for improved in-gust flight of flapping wing MAVs.Front Robot AI. 2022 Dec 7;9:1060933. doi: 10.3389/frobt.2022.1060933. eCollection 2022. Front Robot AI. 2022. PMID: 36569593 Free PMC article.
-
Passive mechanisms in flying insects and applications in bio-inspired flapping-wing micro air vehicles.Proc Biol Sci. 2025 Jul;292(2050):20251015. doi: 10.1098/rspb.2025.1015. Epub 2025 Jul 2. Proc Biol Sci. 2025. PMID: 40592458 Review.
-
Complex hemolymph circulation patterns in grasshopper wings.Commun Biol. 2023 Mar 23;6(1):313. doi: 10.1038/s42003-023-04651-2. Commun Biol. 2023. PMID: 36959465 Free PMC article.
References
-
- Albert P.J., Zacharuk R.Y., Wong L. Structure, innervation, and distribution of sensilla on the wings of a grasshopper. Can. J. Zool. 1976;54 doi: 10.1139/z76-178. 1542–53. - DOI
Associated data
LinkOut - more resources
Full Text Sources

