The T-Type Calcium Channel Cav3.2 in Somatostatin Interneurons in Spinal Dorsal Horn Participates in Mechanosensation and Mechanical Allodynia in Mice
- PMID: 35465611
- PMCID: PMC9024096
- DOI: 10.3389/fncel.2022.875726
The T-Type Calcium Channel Cav3.2 in Somatostatin Interneurons in Spinal Dorsal Horn Participates in Mechanosensation and Mechanical Allodynia in Mice
Abstract
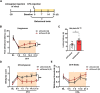
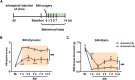
Somatostatin-positive (SOM+) neurons have been proposed as one of the key populations of excitatory interneurons in the spinal dorsal horn involved in mechanical pain. However, the molecular mechanism for their role in pain modulation remains unknown. Here, we showed that the T-type calcium channel Cav3.2 was highly expressed in spinal SOM+ interneurons. Colocalization of Cacna1h (which codes for Cav3.2) and SOM tdTomato was observed in the in situ hybridization studies. Fluorescence-activated cell sorting of SOM tdTomato cells in spinal dorsal horn also proved a high expression of Cacna1h in SOM+ neurons. Behaviorally, virus-mediated knockdown of Cacna1h in spinal SOM+ neurons reduced the sensitivity to light touch and responsiveness to noxious mechanical stimuli in naïve mice. Furthermore, knockdown of Cacna1h in spinal SOM+ neurons attenuated thermal hyperalgesia and dynamic allodynia in the complete Freund's adjuvant-induced inflammatory pain model, and reduced both dynamic and static allodynia in a neuropathic pain model of spared nerve injury. Mechanistically, a decrease in the percentage of neurons with Aβ-eEPSCs and Aβ-eAPs in superficial dorsal horn was observed after Cacna1h knockdown in spinal SOM+ neurons. Altogether, our results proved a crucial role of Cav3.2 in spinal SOM+ neurons in mechanosensation under basal conditions and in mechanical allodynia under pathological pain conditions. This work reveals a molecular basis for SOM+ neurons in transmitting mechanical pain and shows a functional role of Cav3.2 in tactile and pain processing at the level of spinal cord in addition to its well-established peripheral role.
Keywords: SOM neurons; intraspinal injection; knockdown; low-voltage activated calcium channel; spinal cord slice recording.
Copyright © 2022 Zhi, Cao, Su, Gao, Zheng, Jiang, Su, Liu, Wang, Zhang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Nerve injury elevates functional Cav3.2 channels in superficial spinal dorsal horn.Mol Pain. 2019 Jan-Dec;15:1744806919836569. doi: 10.1177/1744806919836569. Mol Pain. 2019. PMID: 30803310 Free PMC article.
-
Cdk5-Dependent Phosphorylation of CaV3.2 T-Type Channels: Possible Role in Nerve Ligation-Induced Neuropathic Allodynia and the Compound Action Potential in Primary Afferent C Fibers.J Neurosci. 2020 Jan 8;40(2):283-296. doi: 10.1523/JNEUROSCI.0181-19.2019. Epub 2019 Nov 19. J Neurosci. 2020. PMID: 31744861 Free PMC article.
-
A subset of spinal dorsal horn interneurons crucial for gating touch-evoked pain-like behavior.Proc Natl Acad Sci U S A. 2021 Jan 19;118(3):e2021220118. doi: 10.1073/pnas.2021220118. Proc Natl Acad Sci U S A. 2021. PMID: 33431693 Free PMC article.
-
PKCγ interneurons, a gateway to pathological pain in the dorsal horn.J Neural Transm (Vienna). 2020 Apr;127(4):527-540. doi: 10.1007/s00702-020-02162-6. Epub 2020 Feb 27. J Neural Transm (Vienna). 2020. PMID: 32108249 Review.
-
Voltage-dependent CaV3.2 and CaV2.2 channels in nociceptive pathways.Pflugers Arch. 2022 Apr;474(4):421-434. doi: 10.1007/s00424-022-02666-y. Epub 2022 Jan 18. Pflugers Arch. 2022. PMID: 35043234 Review.
Cited by
-
An electrophysiologist's guide to dorsal horn excitability and pain.Front Cell Neurosci. 2025 Apr 2;19:1548252. doi: 10.3389/fncel.2025.1548252. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40241846 Free PMC article. Review.
-
Neuroanatomical and neurochemical atlas of the spiny mouse (Acomys cahirinus) spinal cord.Brain Struct Funct. 2025 Jul 28;230(7):124. doi: 10.1007/s00429-025-02982-w. Brain Struct Funct. 2025. PMID: 40719809
-
T-type calcium channel modulation by hydrogen sulfide in neuropathic pain conditions.Front Pharmacol. 2023 Jul 17;14:1212800. doi: 10.3389/fphar.2023.1212800. eCollection 2023. Front Pharmacol. 2023. PMID: 37529702 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

