Comparison of a novel automated DiaSys procalcitonin immunoassay with four different BRAHMS-partnered immunoassays
- PMID: 35465623
- PMCID: PMC9026940
- DOI: 10.1016/j.plabm.2022.e00274
Comparison of a novel automated DiaSys procalcitonin immunoassay with four different BRAHMS-partnered immunoassays
Abstract
Objectives: Procalcitonin (PCT) is an important biomarker of sepsis and respiratory infections. Various automated immunoassays for measuring PCT in patient plasma are available in medical laboratories. However, due to a lack of international reference material for PCT, the assays are not always comparable.
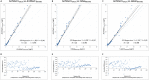
Design and methods: In this study, we compared a new turbidimetric immunoassay from DiaSys, measured on the Abbott Architect c16000 and Alinity c, with four BRAHMS-associated chemiluminescence immunoassays (Abbott Architect i2000SR, Alinity i, Roche Cobas e411 and DiaSorin Liaison XL) using 120 random patient plasma samples from the clinical laboratory routine at the University Medical Center Goettingen.
Results: The DiaSys assay showed clear differences as compared to the BRAHMS-associated assays when measured on Architect c: i.e. 58% positive mean bias vs. Architect i, 67% vs. Cobas and 23% vs. Liaison. As a result, additional 19% our patients would have a suspected bacterial infection, when using PCT values from the DiaSys assay and commonly accepted decision limits. A crosscheck of the DiaSys calibrator on the BRAHMS-associated systems showed a low recovery of the calibrator material (approx. 50%).
Conclusions: Overall, this study shows significant differences between the DiaSys and BRAHMS-associated assays. This could be attributed to a potential DiaSys calibrator problem. This highlights the need for an international reference material for harmonization of the PCT assays.
Keywords: Abbott; Alinity; Architect; Bacterial infection; Calibration; Cobas; Comparison; DiaSys; Diasorin; Immunoassay; Liaison; PCT; PETIA; Procalcitonin; Reference material; Roche; Sepsis; Standardization.
© 2022 The Authors.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

Similar articles
-
Multicenter comparison of automated procalcitonin immunoassays.Pract Lab Med. 2015 Jul 17;2:22-28. doi: 10.1016/j.plabm.2015.07.001. eCollection 2015 Aug 1. Pract Lab Med. 2015. PMID: 28932801 Free PMC article.
-
Two-center comparison of 10 fully-automated commercial procalcitonin (PCT) immunoassays.Clin Chem Lab Med. 2019 Dec 18;58(1):77-84. doi: 10.1515/cclm-2019-0888. Clin Chem Lab Med. 2019. PMID: 31539351
-
National external quality assessment and direct method comparison reflect crucial deviations of Procalcitonin measurements in Germany.Clin Chim Acta. 2022 Apr 1;529:67-75. doi: 10.1016/j.cca.2022.02.007. Epub 2022 Feb 12. Clin Chim Acta. 2022. PMID: 35167843
-
Evaluation of procalcitonin immunoassay concordance near clinical decision points.Clin Chem Lab Med. 2019 Aug 27;57(9):1414-1421. doi: 10.1515/cclm-2018-1362. Clin Chem Lab Med. 2019. PMID: 30763263
-
Harmonization status of procalcitonin measurements: what do comparison studies and EQA schemes tell us?Clin Chem Lab Med. 2021 Jun 21;59(10):1610-1622. doi: 10.1515/cclm-2021-0566. Print 2021 Sep 27. Clin Chem Lab Med. 2021. PMID: 34147043 Review.
Cited by
-
Comment to: Extensive analytical evaluation of the performances of the new DiaSys PCT assay and comparison with Elecsys B·R·A·H·M·S PCT test on Roche Cobas and B·R·A·H·M·S PCT-sensitive Kryptor.Clin Chem Lab Med. 2023 Sep 26;62(2):e48-e50. doi: 10.1515/cclm-2023-0997. Print 2024 Jan 26. Clin Chem Lab Med. 2023. PMID: 37746855 No abstract available.
References
LinkOut - more resources
Full Text Sources
Research Materials

