Neural plasticity of the uterus: New targets for endometrial cancer?
- PMID: 35465787
- PMCID: PMC9047769
- DOI: 10.1177/17455057221095537
Neural plasticity of the uterus: New targets for endometrial cancer?
Abstract
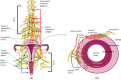
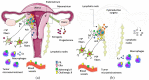
Endometrial carcinoma is the most common gynecological malignancy in Western countries and is expected to increase in the following years because of the high index of obesity in the population. Recently, neural signaling has been recognized as part of the tumor microenvironment, playing an active role in tumor progression and invasion of different solid tumor types. The uterus stands out for the physiological plasticity of its peripheral nerves due to cyclic remodeling brought on by estrogen and progesterone hormones throughout the reproductive cycle. Therefore, a precise understanding of nerve-cancer crosstalk and the contribution of the organ-intrinsic neuroplasticity, mediated by estrogen and progesterone, of the uterine is urgently needed. The development of new and innovative medicines for patients with endometrial cancer would increase their quality of life and health. This review compiles information on the architecture and function of autonomous uterine neural innervations and the influence of hormone-dependent nerves in normal uterus and tumor progression. It also explores new therapeutic possibilities for endometrial cancer using these endocrine and neural advantages.
Keywords: autonomic nervous system; endometrial cancer; nerve plasticity; nerve-cancer; neuroreceptor; neurotherapy; neurotransmitter; sex hormones signaling.
Conflict of interest statement
Figures
References
-
- Silverman JE, Gulati A. An overview of interventional strategies for the management of oncologic pain. Pain Manag 2018; 8(5): 389–403. - PubMed
-
- Amit M, Na’ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nat Rev Cancer 2016; 16(6): 399–408. - PubMed
-
- Pundavela J, Demont Y, Jobling P, et al.. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am J Pathol 2014; 184(12): 3156–3162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

