Antipseudomonal treatment decisions during CF exacerbation management
- PMID: 35466039
- PMCID: PMC9509480
- DOI: 10.1016/j.jcf.2022.04.006
Antipseudomonal treatment decisions during CF exacerbation management
Abstract
Background: Cystic fibrosis (CF) pulmonary exacerbation (PEx) treatment guidelines suggest that Pseudomonas aeruginosa (Pa) airway infection be treated with two antipseudomonal agents.
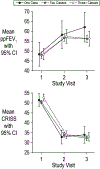
Methods: We retrospectively studied treatment responses for STOP2 PEx treatment trial (NCT02781610) participants with a history of Pa infection. Mean lung function and symptom changes from intravenous (IV) antimicrobial treatment start to Visit 2 (7 to 10 days later) were compared between those receiving one, two, and three+ antipseudomonal classes before Visit 2 by ANCOVA. Odds of PEx retreatment with IV antimicrobials within 30 days and future IV-treated PEx hazard were modeled by logistic and Cox proportional hazards regression, respectively. Sensitivity analyses limited to the most common one-, two-, and three-class regimens, to only IV/oral antipseudomonal treatments, and with more stringent Pa infection definitions were conducted.
Results: Among 751 participants, 50 (6.7%) were treated with one antipseudomonal class before Visit 2, while 552 (73.5%) and 149 (19.8%) were treated with two and with three+ classes, respectively. Females and participants with a negative Pa culture in the prior month were more likely to be treated with a single class. The most common single, double, and triple class regimens were beta-lactam (BL; n = 42), BL/aminoglycoside (AG; n = 459), and BL/AG/fluoroquinolone (FQ; n = 73). No lung function or symptom response, odds of retreatment, or future PEx hazard differences were observed by number of antipseudomonal classes administered in primary or sensitivity analyses.
Conclusions: We were unable to identify additional benefit when multiple antipseudomonal classes are used to treat PEx in people with CF and Pa.
Keywords: Antipseudomonal classes; P. aeruginosa; Pulmonary exacerbation.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Conflicts of interest
The authors claim no financial conflicts of interest related to this work.
Figures

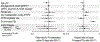
References
-
- Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl ME, Wagener JS, Regelmann WE, Johnson CA; Scientific Advisory Group and the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007. Aug;151(2):134–9, 139.e1. - PubMed
-
- Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002; 166(12 Pt 1):1550–5. - PubMed
-
- Ratjen F, Döring G. Cystic fibrosis. Lancet. 2003. Feb 22;361(9358):681–9. Review. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

