Improving Recruitment for a Newborn Screening Pilot Study with Adaptations in Response to the COVID-19 Pandemic
- PMID: 35466194
- PMCID: PMC9036248
- DOI: 10.3390/ijns8020023
Improving Recruitment for a Newborn Screening Pilot Study with Adaptations in Response to the COVID-19 Pandemic
Abstract
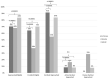
Seven months after the launch of a pilot study to screen newborns for Duchenne Muscular Dystrophy (DMD) in New York State, New York City became an epicenter of the coronavirus disease 2019 (COVID-19) pandemic. All in-person research activities were suspended at the study enrollment institutions of Northwell Health and NewYork-Presbyterian Hospitals, and study recruitment was transitioned to 100% remote. Pre-pandemic, all recruitment was in-person with research staff visiting the postpartum patients 1-2 days after delivery to obtain consent. With the onset of pandemic, the multilingual research staff shifted to calling new mothers while they were in the hospital or shortly after discharge, and consent was collected via emailed e-consent links. With return of study staff to the hospitals, a hybrid approach was implemented with in-person recruitment for babies delivered during the weekdays and remote recruitment for babies delivered on weekends and holidays, a cohort not recruited pre-pandemic. There was a drop in the proportion of eligible babies enrolled with the transition to fully remote recruitment from 64% to 38%. In addition, the proportion of babies enrolled after being approached dropped from 91% to 55%. With hybrid recruitment, the proportion of eligible babies enrolled (70%) and approached babies enrolled (84%) returned to pre-pandemic levels. Our experience adapting our study during the COVID-19 pandemic led us to develop new recruitment strategies that we continue to utilize. The lessons learned from this pilot study can serve to help other research studies adapt novel and effective recruitment methods.
Keywords: COVID-19 pandemic; Duchenne Muscular Dystrophy; newborn screening.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Ahimaz P., Giordano J., Disco M., Harrington E., Levinson E., Spiegel E., Andrews C., Griffin E., Hernan R., Wynn J. COVID contingencies: Early epicenter experiences of different genetics clinics at a New York City institution inform emergency adaptation strategies. J. Genet. Couns. 2021;30:938–948. doi: 10.1002/jgc4.1409. - DOI - PMC - PubMed
-
- Moat S., Korpimäki T., Furuk P., Hakala H., Polari H., Meriö L., Mäkinen P., Weeks I. Characterization of a Blood Spot Creatine Kinase Skeletal Muscle Isoform Immunoassay for High-Throughput Newborn Screening of Duchenne Muscular Dystrophy. Clin. Chem. 2017;63:908–914. doi: 10.1373/clinchem.2016.268425. - DOI - PubMed
-
- Nominate a Condition Official Web Site of the U.S. [(accessed on 6 December 2021)]; Health Resources & Services Administration. Available online: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/nomina....
LinkOut - more resources
Full Text Sources

