ZSCAN1 Autoantibodies Are Associated with Pediatric Paraneoplastic ROHHAD
- PMID: 35466441
- PMCID: PMC9329235
- DOI: 10.1002/ana.26380
ZSCAN1 Autoantibodies Are Associated with Pediatric Paraneoplastic ROHHAD
Abstract
Objective: Rapid-onset Obesity with Hypothalamic Dysfunction, Hypoventilation and Autonomic Dysregulation (ROHHAD), is a severe pediatric disorder of uncertain etiology resulting in hypothalamic dysfunction and frequent sudden death. Frequent co-occurrence of neuroblastic tumors have fueled suspicion of an autoimmune paraneoplastic neurological syndrome (PNS); however, specific anti-neural autoantibodies, a hallmark of PNS, have not been identified. Our objective is to determine if an autoimmune paraneoplastic etiology underlies ROHHAD.
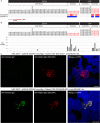
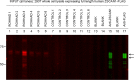
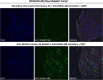
Methods: Immunoglobulin G (IgG) from pediatric ROHHAD patients (n = 9), non-inflammatory individuals (n = 100) and relevant pediatric controls (n = 25) was screened using a programmable phage display of the human peptidome (PhIP-Seq). Putative ROHHAD-specific autoantibodies were orthogonally validated using radioactive ligand binding and cell-based assays. Expression of autoantibody targets in ROHHAD tumor and healthy brain tissue was assessed with immunohistochemistry and mass spectrometry, respectively.
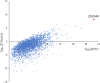
Results: Autoantibodies to ZSCAN1 were detected in ROHHAD patients by PhIP-Seq and orthogonally validated in 7/9 ROHHAD patients and 0/125 controls using radioactive ligand binding and cell-based assays. Expression of ZSCAN1 in ROHHAD tumor and healthy human brain tissue was confirmed.
Interpretation: Our results support the notion that tumor-associated ROHHAD syndrome is a pediatric PNS, potentially initiated by an immune response to peripheral neuroblastic tumor. ZSCAN1 autoantibodies may aid in earlier, accurate diagnosis of ROHHAD syndrome, thus providing a means toward early detection and treatment. This work warrants follow-up studies to test sensitivity and specificity of a novel diagnostic test. Last, given the absence of the ZSCAN1 gene in rodents, our study highlights the value of human-based approaches for detecting novel PNS subtypes. ANN NEUROL 2022;92:279-291.
© 2022 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

